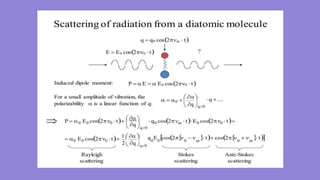
Raman spectroscopy is an analytical technique that uses scattered light to measure the vibrational energy modes of a sample. It was discovered in 1928 by C.V. Raman and K.S. Krishnan, for which Raman won the 1930 Nobel Prize. Raman scattering occurs when light interacts with molecular vibrations, resulting in the energy of the scattered photons being shifted up or down. This provides information about the chemical structure and bonds within a sample. The shift is measured as a Raman spectrum that is unique for different materials.