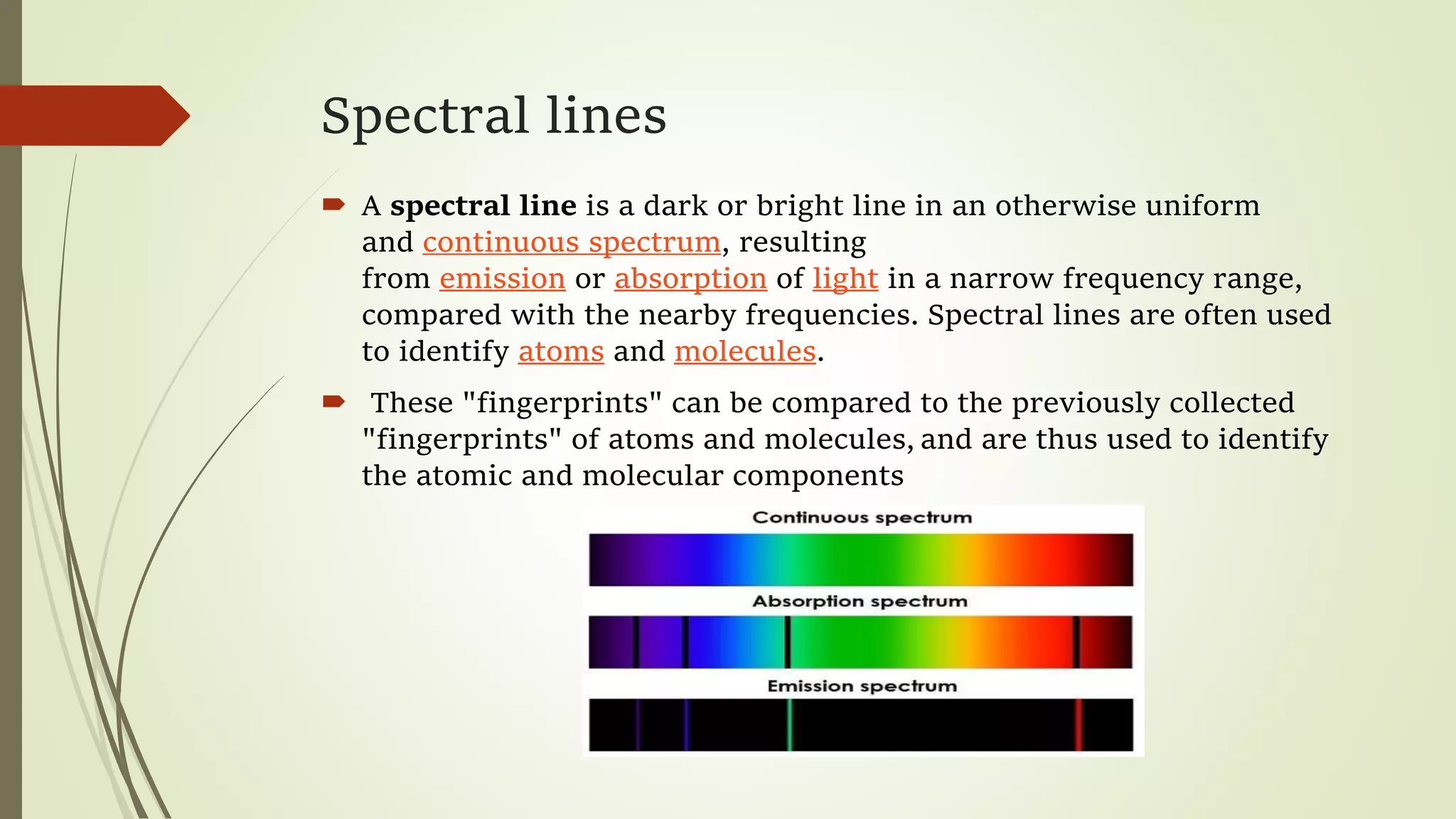
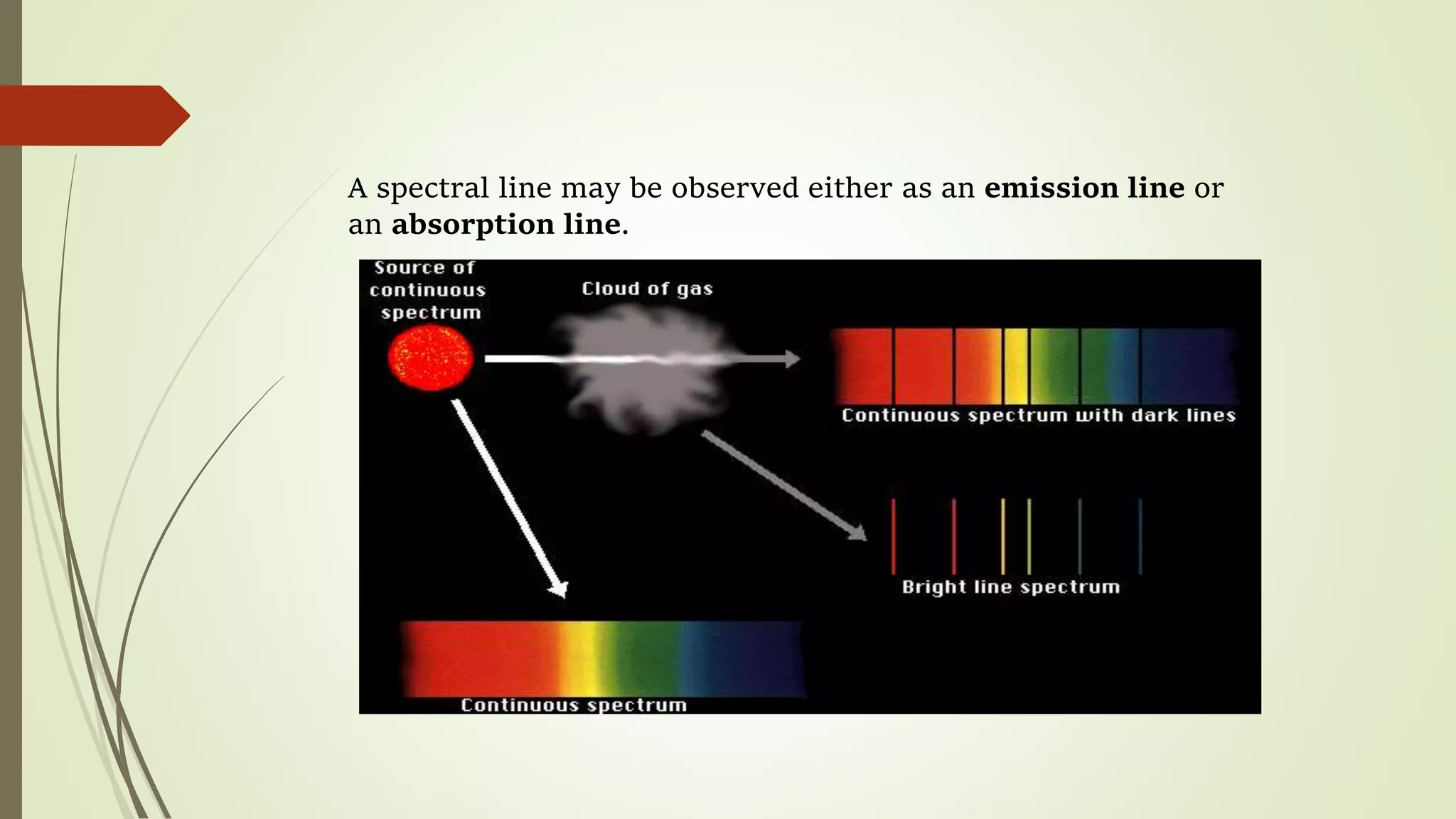
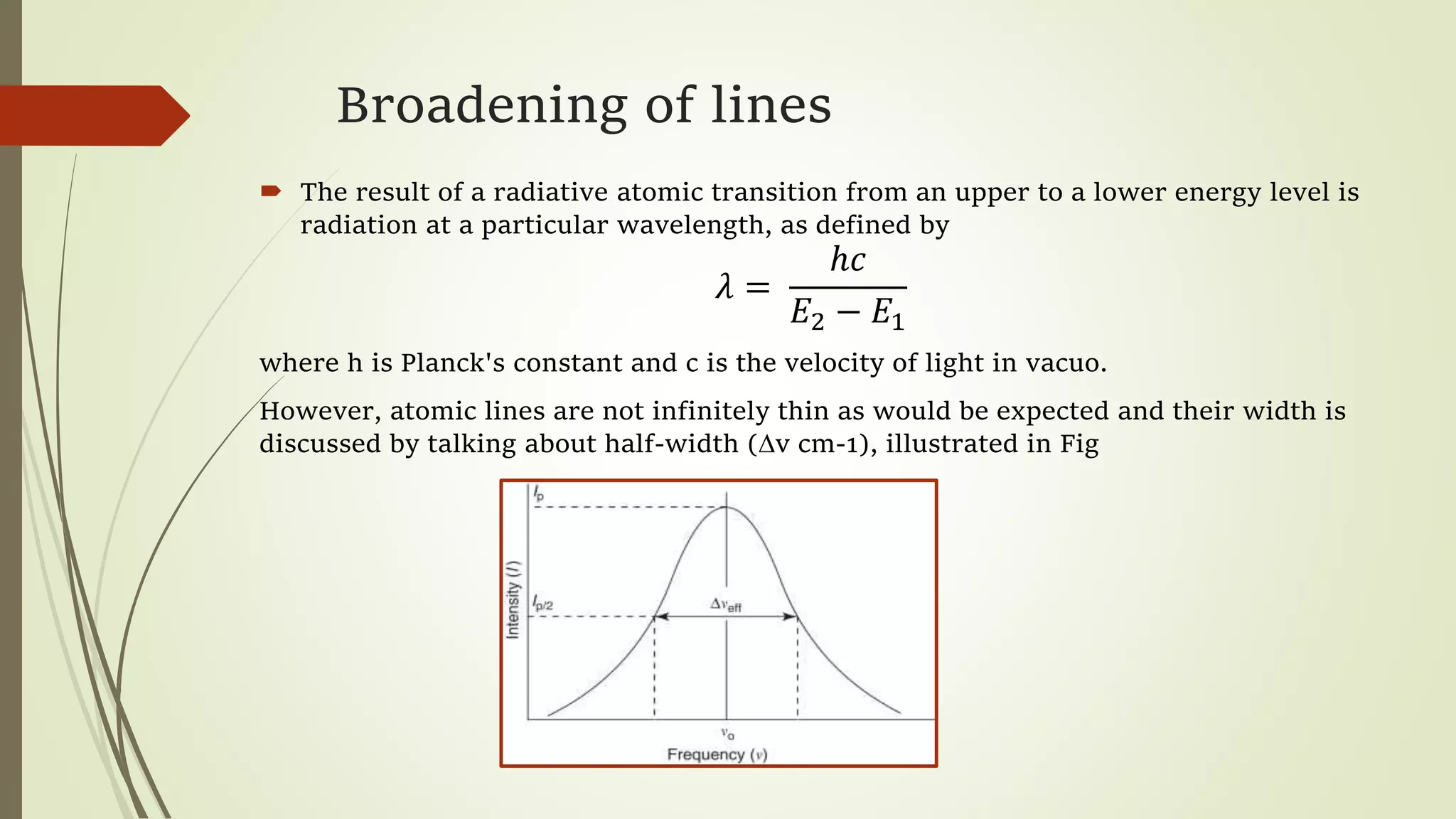
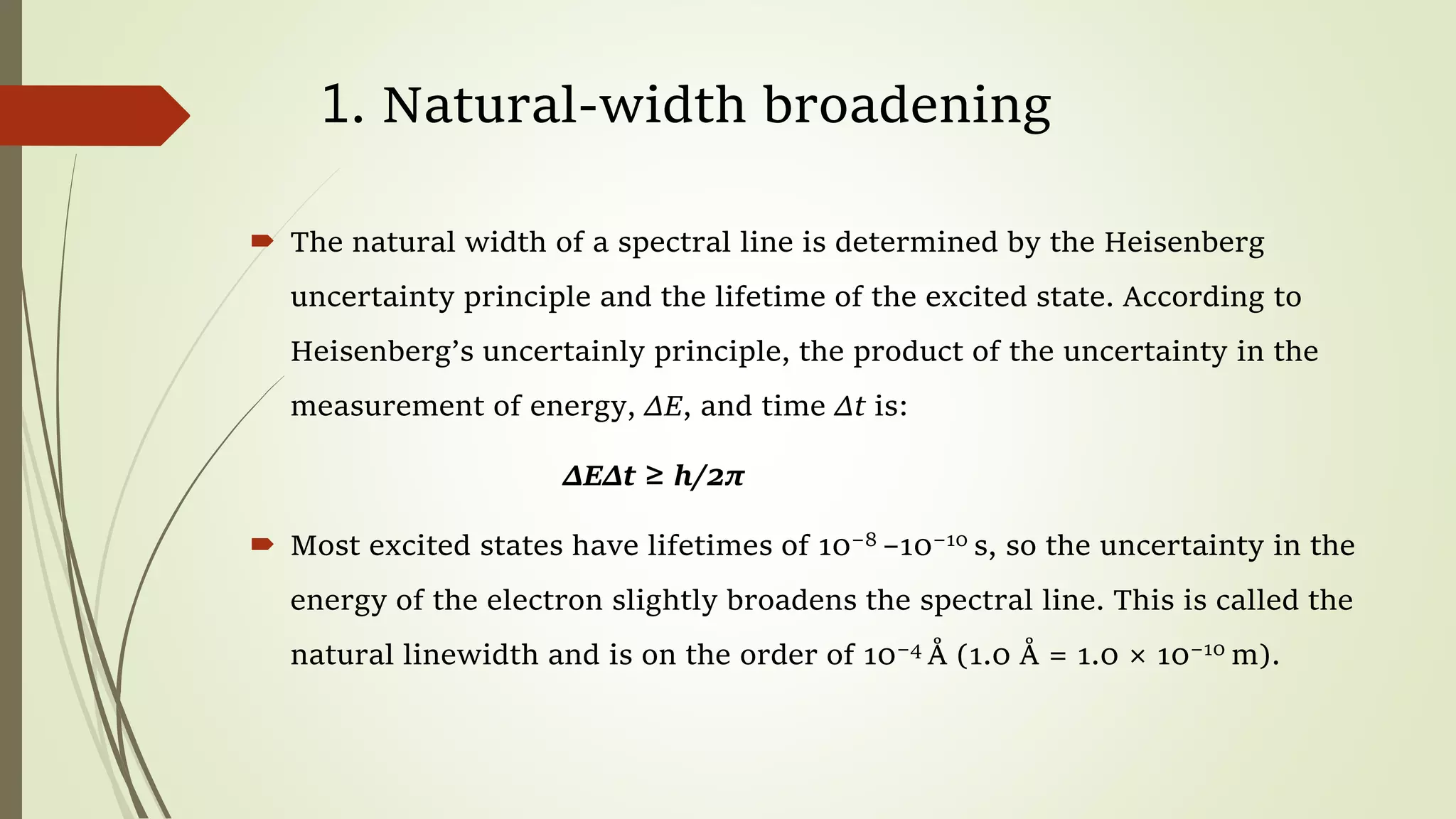
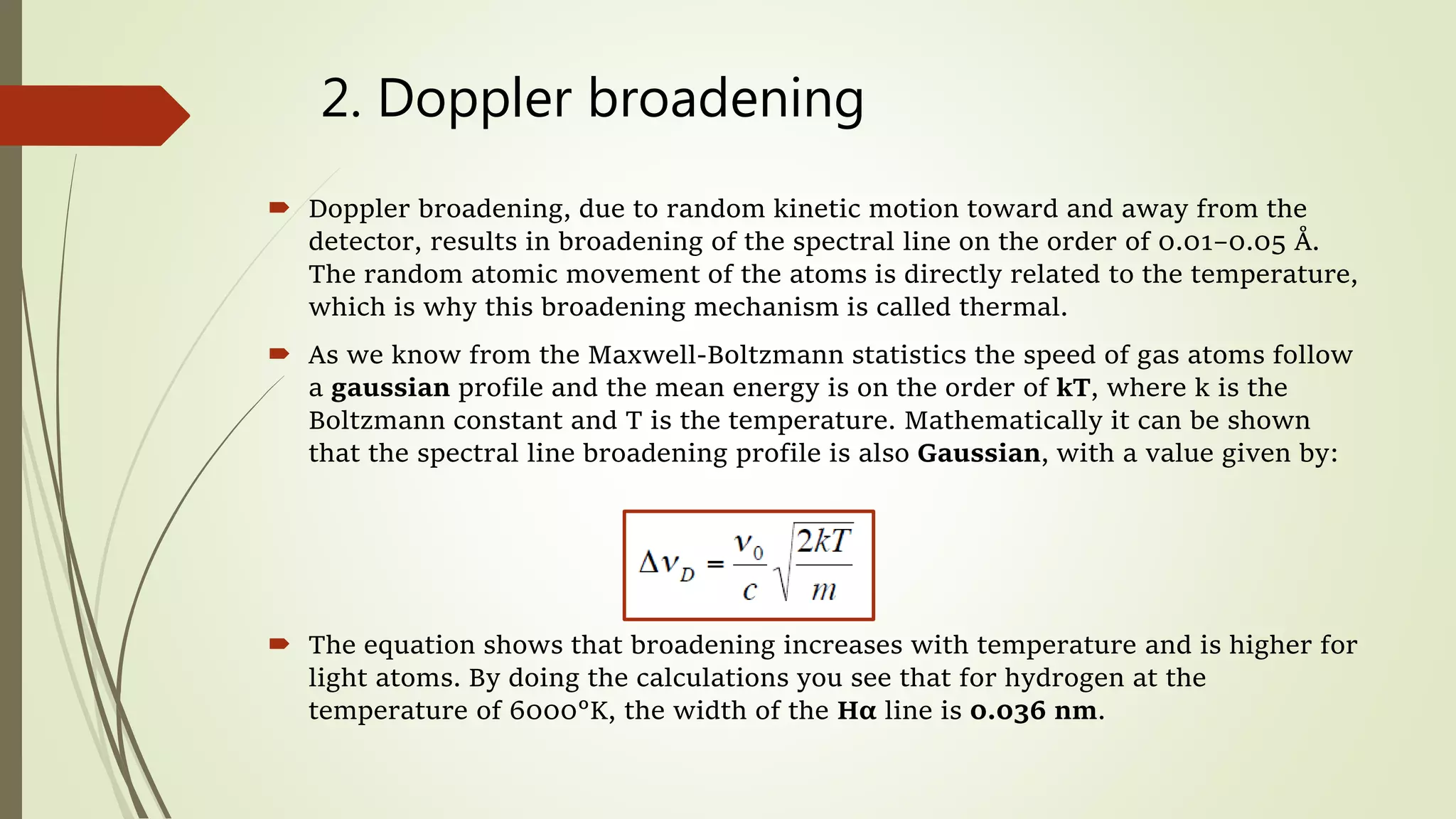
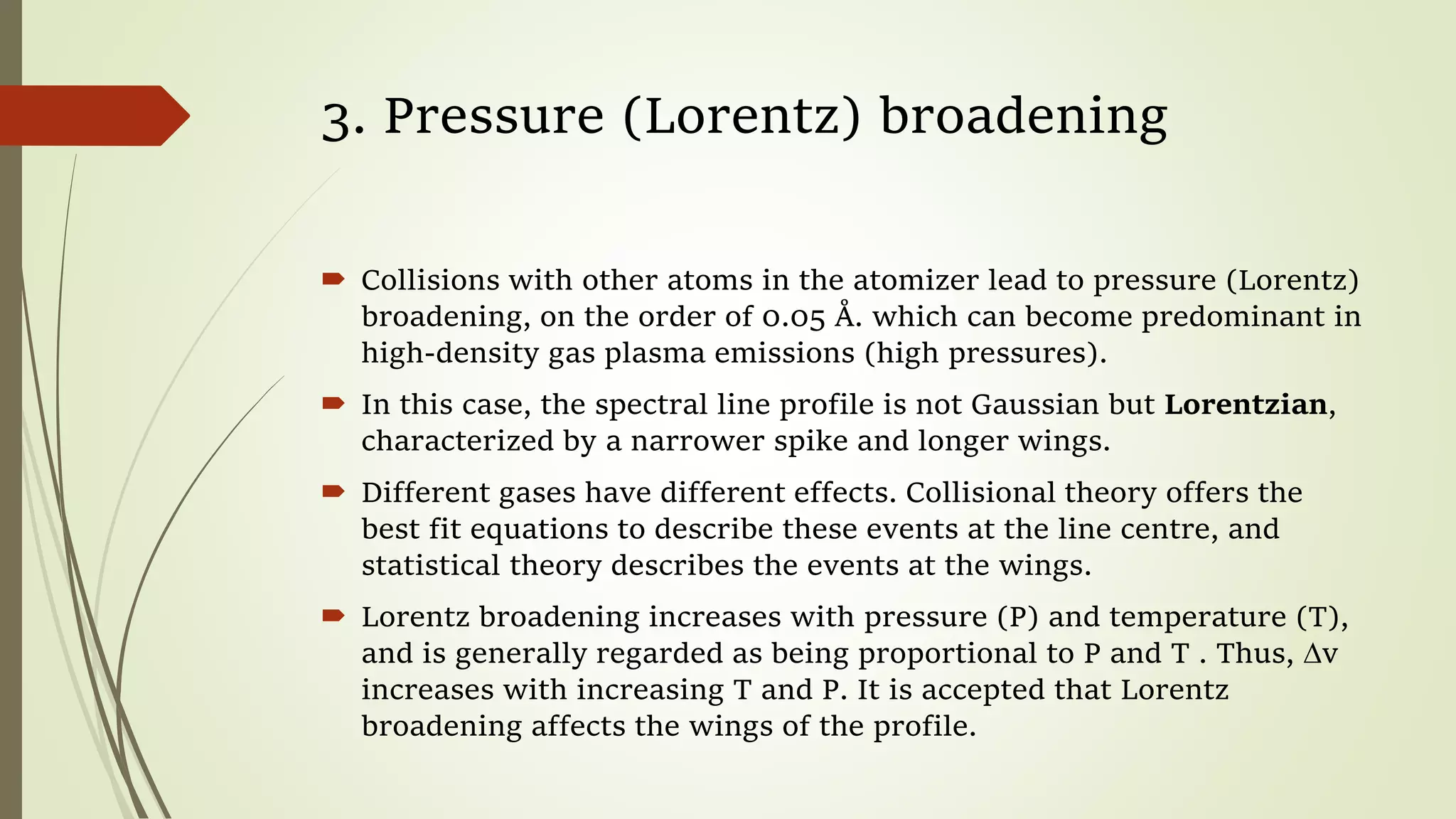
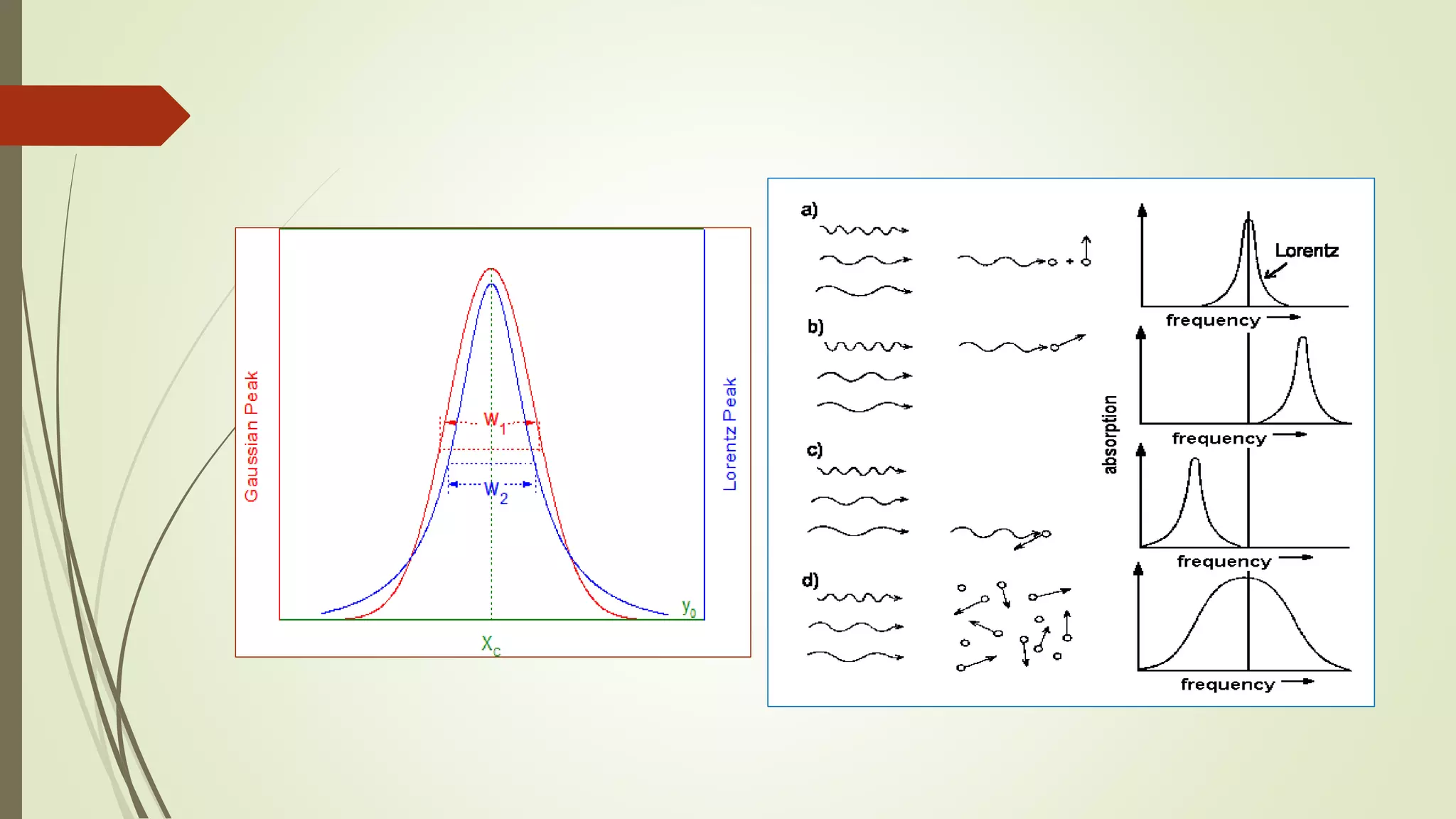
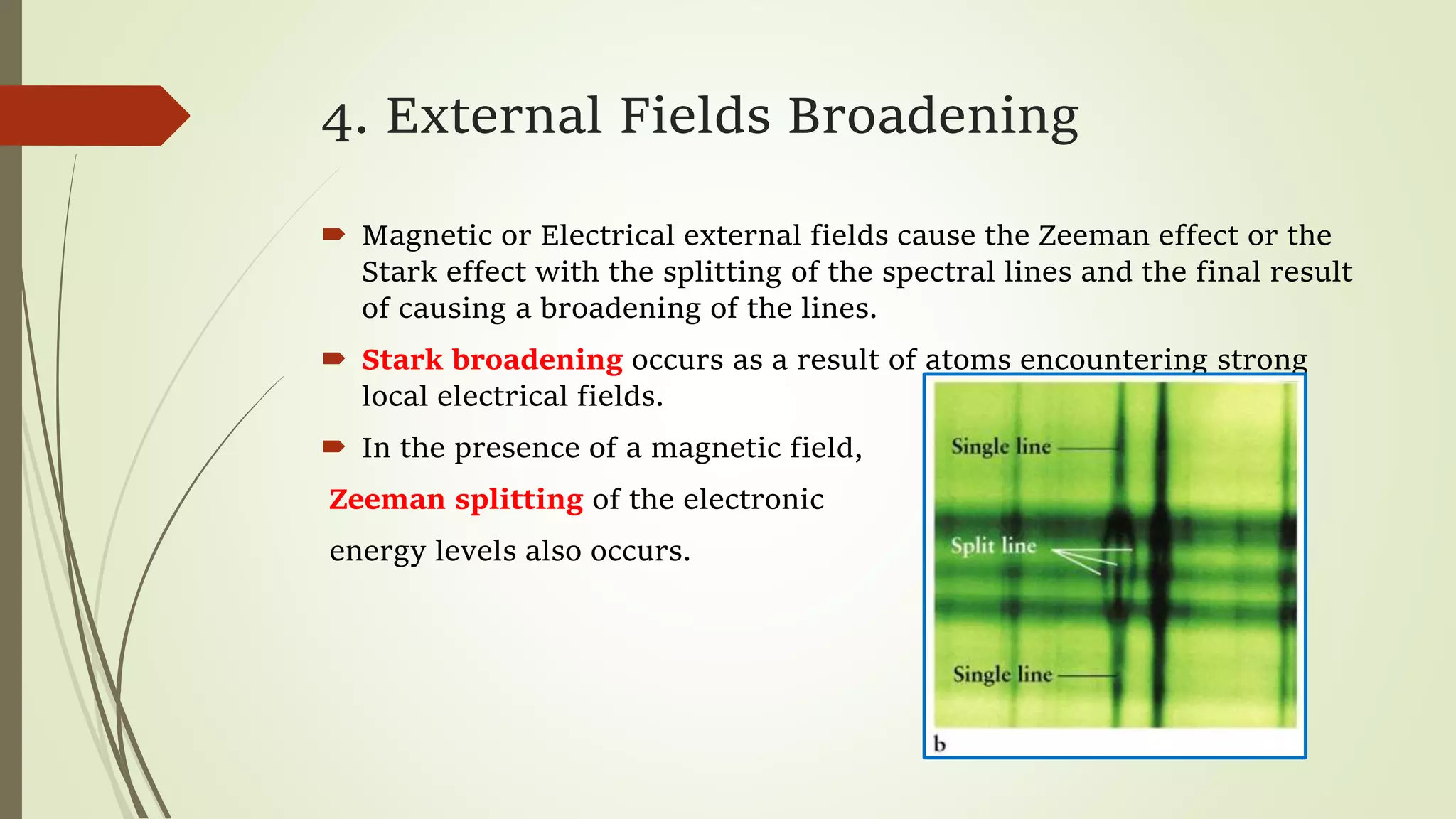
This lecture discusses spectral lines and broadening. A spectral line results from the absorption or emission of light at a narrow frequency range, which can be used to identify atoms and molecules. Spectral lines experience natural broadening due to the Heisenberg uncertainty principle. Doppler broadening occurs due to the random motion of atoms and increases with temperature. Pressure or Lorentz broadening results from atomic collisions and increases with pressure and temperature. External magnetic or electric fields can also cause Zeeman or Stark broadening through the splitting of spectral lines.