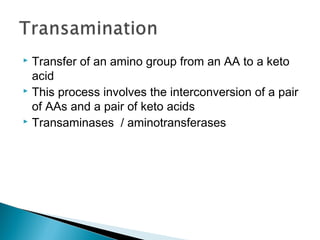
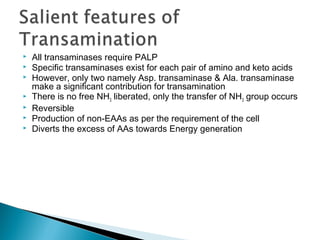
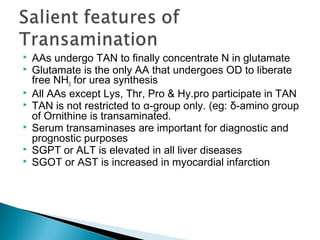
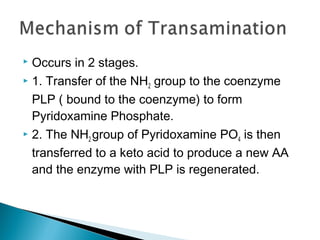
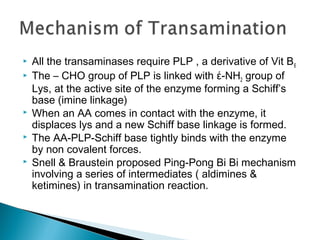
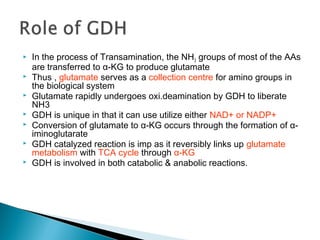
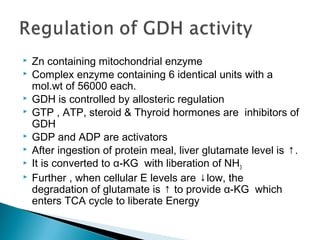
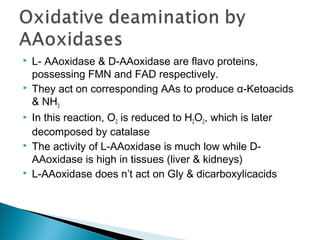
This document discusses protein metabolism and the metabolism of amino acids. It notes that proteins are the most abundant organic compounds in the body, making up 10-12kg of dry weight, and perform many structural and functional roles. Amino acids are the building blocks of proteins and there are 20 naturally occurring amino acids, with 10 being non-essential and needing to be synthesized by the body. The amino acid pool is maintained through intake from dietary proteins and synthesis of non-essential amino acids, and is utilized for protein synthesis and other metabolic processes. Protein metabolism involves the constant breakdown and resynthesis of body proteins.