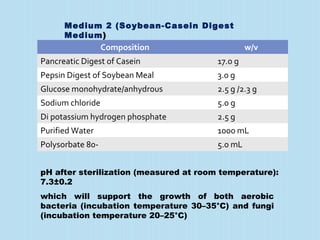
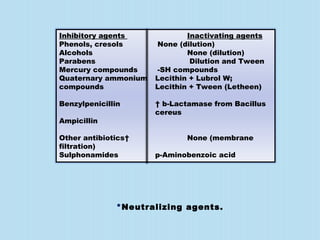
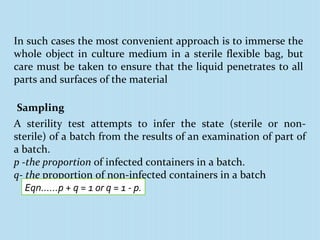
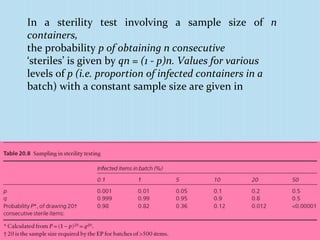
A sterility test assesses whether a pharmaceutical product is free from microorganisms. It involves incubating samples of the product in nutrient media. There are three main methods for conducting sterility tests: direct inoculation into media, membrane filtration, and adding concentrated media to products in their original containers. Two common media used are fluid thioglycollate medium and soybean-casein digest medium. Controls and appropriate sampling methods are necessary to accurately determine if a batch meets sterility requirements.