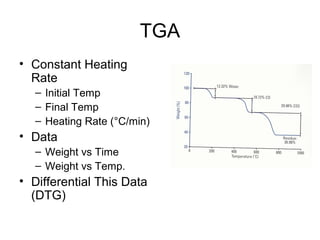
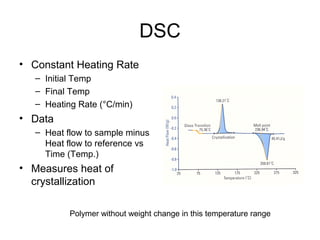
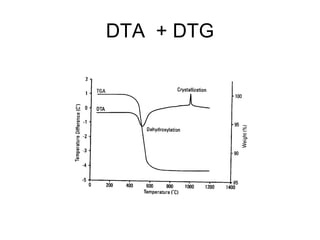
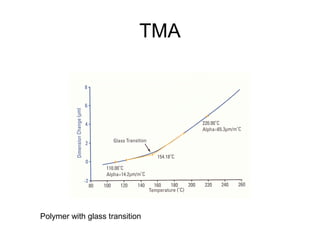
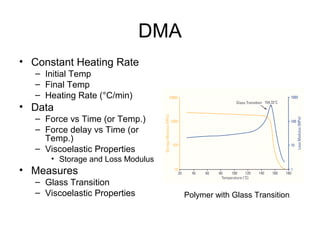
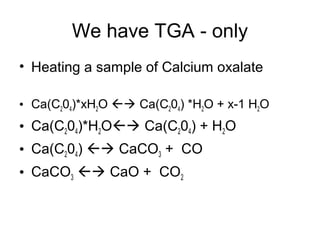
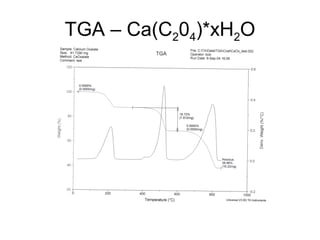
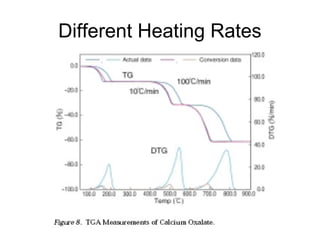
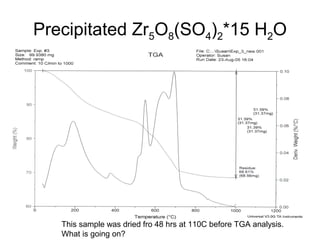
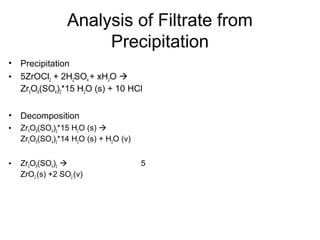
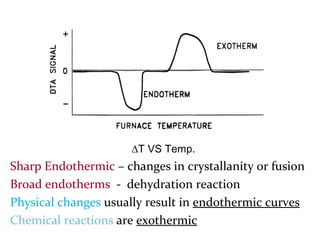
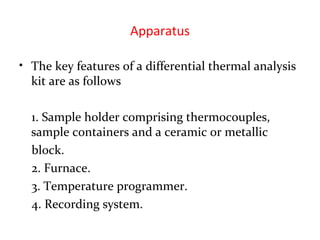
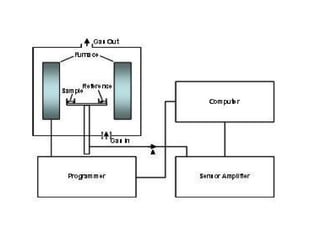
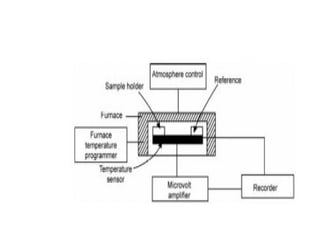
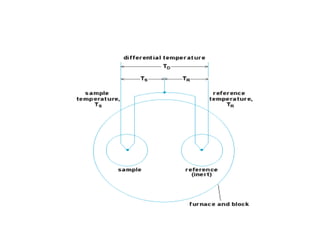
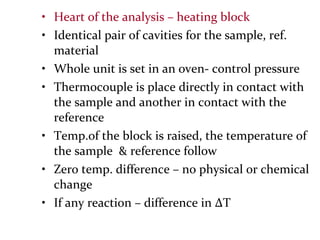
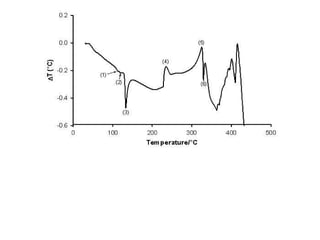
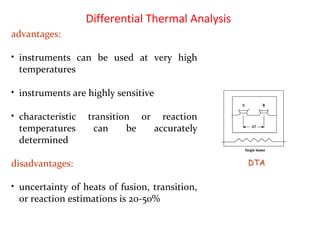
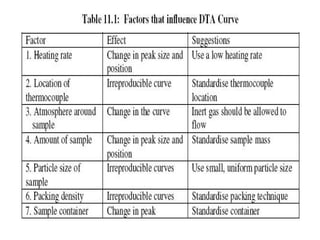
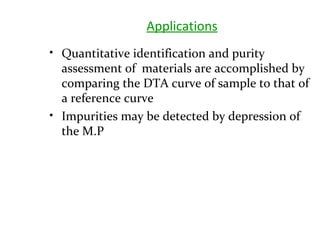
This document discusses several thermal analysis techniques including differential thermal analysis (DTA). It explains that DTA involves heating a sample and inert reference material simultaneously and measuring any temperature difference, which can indicate physical or chemical changes in the sample. The document provides details on DTA instrumentation, the factors that can affect DTA results, and applications such as material identification and purity assessment by comparing DTA curves.