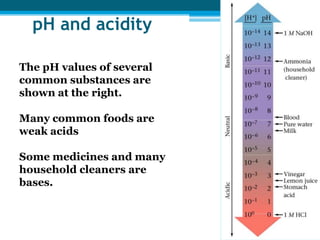
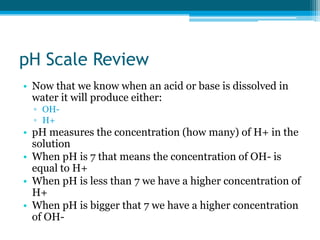
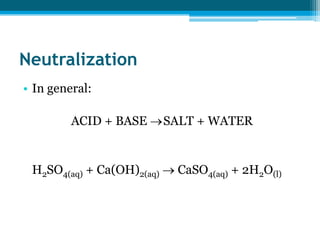
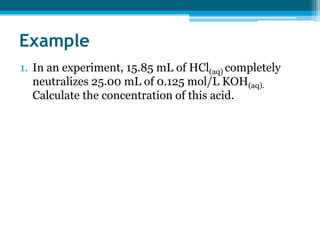
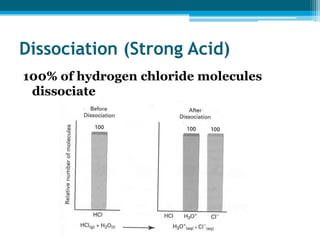
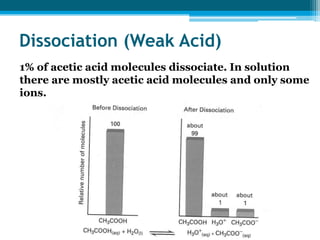
This document discusses acids and bases. It defines strong acids as acids that dissociate completely in water, such as HCl, while weak acids like acetic acid dissociate only slightly. The concentration of H3O+ ions in strong acids equals the concentration of the acid. In weak acids it is much less. Strong bases like NaOH also dissociate completely while weak bases like ammonia dissociate slightly. pH is a measure of H3O+ concentration and indicates if a solution is acidic (pH < 7) or basic (pH > 7). Acid-base reactions involve neutralization of acids by bases to form salts and water. Titration can be used to determine unknown concentrations using indicators and the





![Concentration
• the concentration of H3O+(aq) ions, [H3O+],
in a dilute solution of a SA is equal to the
concentration of the acid, [acid]
[H3O+] = [strong acid]
• Ex. In a sample of 1.0 mol/L HCl(aq)
[H3O+] = 1.0 mol/L
Recall: C = n
square bracket V
Molar Concentration](https://image.slidesharecdn.com/4-acidsbases-130103233004-phpapp01/85/4-acids-bases-10-320.jpg)
![Concentration
• the concentration of H3O+(aq) ions, [H3O+],
in a dilute solution of a WA is much less
than the concentration of the acid, [acid]
• Ex. In a sample of 1.0 mol/L CH3COOH (aq)
[H3O+] <<< 1.0 mol/L
• That is, [H3O+] < [weak acid]](https://image.slidesharecdn.com/4-acidsbases-130103233004-phpapp01/85/4-acids-bases-11-320.jpg)

![Weak Base (WB)
• most bases are weak
• WB dissociates very slightly into ions in
water
▫ Ex. Ammonia [OH1-] < [weak base]](https://image.slidesharecdn.com/4-acidsbases-130103233004-phpapp01/85/4-acids-bases-13-320.jpg)
![Concentration
• the concentration of OH1-(aq) ions, [OH1-],
in a dilute solution of a SB is equal to the
conc. of the base, [base]
[OH1-] = [strong base]
Ex. In a sample of 1.0 mol/L NaOH(aq)
[OH1-] = 1.0 mol/L](https://image.slidesharecdn.com/4-acidsbases-130103233004-phpapp01/85/4-acids-bases-14-320.jpg)

![16
pH [H3O+ ] [OH- ]
pOH](https://image.slidesharecdn.com/4-acidsbases-130103233004-phpapp01/85/4-acids-bases-16-320.jpg)
![Measuring Strength – pH Scale
• In pure water,
[H3O+] = [OH-] = 1.0 x 10-7 mol/L
• In acidic solution, [H3O+] > [OH-]
• In basic solution, [H3O+] < [OH-]](https://image.slidesharecdn.com/4-acidsbases-130103233004-phpapp01/85/4-acids-bases-17-320.jpg)