Embed presentation
Downloaded 156 times





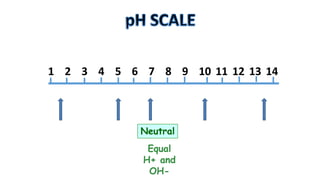
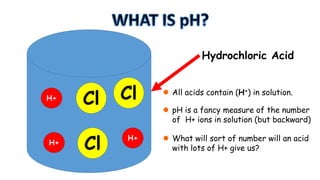
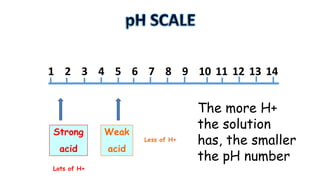
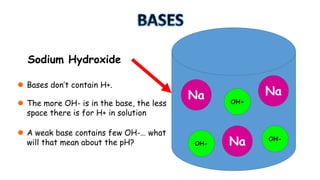
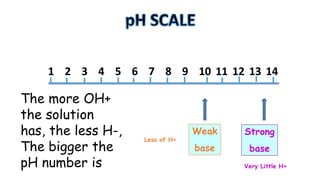



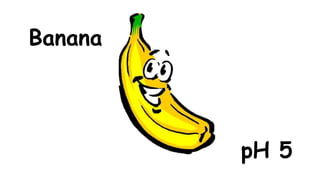
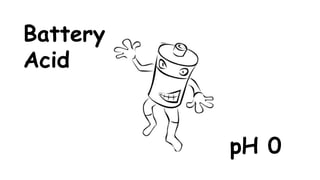
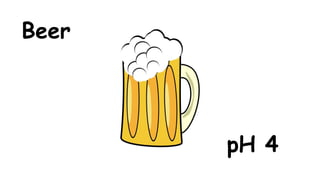


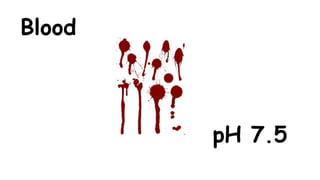


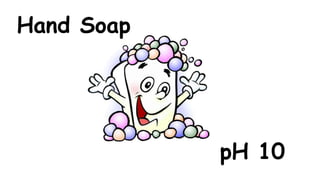
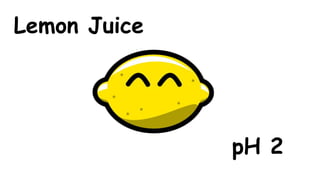
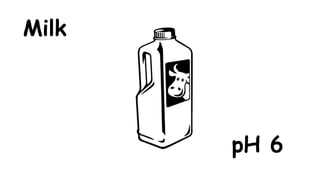
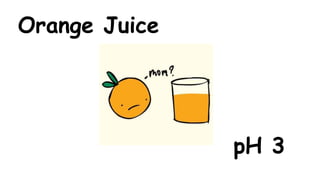
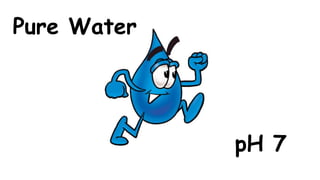
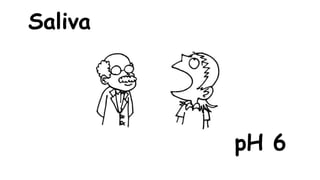
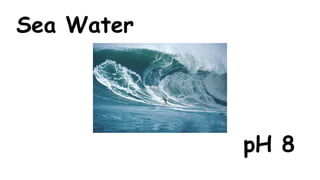
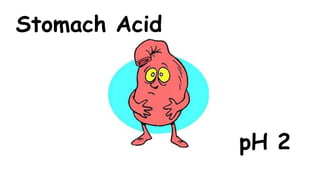
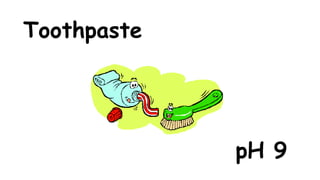


The document provides information about pH and the pH scale. It defines pH as a measure of the concentration of hydrogen ions (H+) in a solution, with lower pH numbers indicating more H+ ions and higher numbers indicating fewer H+ ions. Examples are given of the pH of common household substances like lemon juice, milk, orange juice and others to help understand where different substances fall on the pH scale.






























