This document details an experiment aimed at standardizing a sodium hydroxide (NaOH) solution and determining the concentration of an unknown sulfuric acid (H2SO4) solution. The experiment consists of two main parts: first, NaOH is standardized using potassium hydrogen phthalate (KHP) through titration, resulting in an average concentration of 4.35 M; second, H2SO4 concentration is found to be consistently 1.2 M across trials. It discusses potential sources of experimental error, particularly in measurement accuracy and titration observation.

![Concentration of NaOH = No. of moles / Volume
= [0.0021 mol / {(22.50 + 25) / 1000} L] * 100
= 4.4 M
Trial 2
Mass of KHP transferred = 0.4139 g
Volume of Distilled water = 25 mL
Volume of NaOH used = 22.80 mL
Molar mass of KHP = 204.22 g/mol
No. of moles of KHP = Mass of KHP used / Molar mass
= 0.4139 g / 204.22 g/mol
= 0.0020267 moles
Concentration of NaOH = No. of moles / Volume
= [0.0020267 mol / {(22.80 + 25) / 1000} L] * 100
= 4.24 M
Trial 3
Mass of KHP transferred = 0.4239 g
Volume of Distilled water = 25 mL
Volume of NaOH used = 23.10 mL
Molar mass of KHP = 204.22 g/mol
No. of moles of KHP = Mass of KHP used / Molar mass
= 0.4239 g / 204.22 g/mol
= 0.0020757 moles
Concentration of NaOH = No. of moles / Volume](https://image.slidesharecdn.com/labreport4-131029210010-phpapp02/85/Chemistry-Lab-Report-on-standardization-of-acid-and-bases-2-320.jpg)
![= [0.0020757 mol / {(23.10 + 25) / 1000} L] * 100
= 4.32 M
Trial 4
Mass of KHP transferred = 0.4311 g
Volume of Distilled water = 25 mL
Volume of NaOH used = 22.60 mL
Molar mass of KHP = 204.22 g/mol
No. of moles of KHP = Mass of KHP used / Molar mass
= 0.4311 g / 204.22 g/mol
= 0.0021109 moles
Concentration of NaOH = No. of moles / Volume
= [0.0021109 mol / {(22.60 + 25) / 1000} L] * 100
= 4.43 M
Table:
Trail 1
Mass weighing bottle +
KHP (g)
Mass empty weighing bottle
(g)
Mass of KHP transferred
(g)
Initial volume of burette, Vi
(mL)
Final Volume of burette,
Vf(mL)
Volume of NaOH used
(mL)
Trial 2
Trial 3
Trial 4
11.561
11.6217
11.6113
11.6329
11.1461
11.2078
11.1874
11.2018
0.4200
0.4139
0.4239
0.4311
4.30
6.30
10.1
33.20
26.80
29.10
33.20
55.80
22.50
22.80
23.10
22.60](https://image.slidesharecdn.com/labreport4-131029210010-phpapp02/85/Chemistry-Lab-Report-on-standardization-of-acid-and-bases-3-320.jpg)
![Concentration of NaOH
(moles/L)
4.4
4.24
4.32
Average concentration of NaOH = [4.4 M + 4.24 M + 4.32 M + 4.43 M] / 4
= 4.35 M
1. % Difference between Trial 1 and Trail 2 = [4.24 M / 4.4 M] * 100 %
= 96.3 %
Difference = (100 – 96.3) %
= 3.7 %
2. % Difference between Trial 2 and Trail 3 = [4.24 M / 4.32 M] * 100 %
= 98.1 %
Difference = (100 – 98.1) %
= 1.9 %
3. % Difference between Trial 3 and Trail 4 = [4.32 M / 4.43 M] * 100 %
= 97.5 %
Difference = (100 – 97.5) %
= 2.5 %
4.43](https://image.slidesharecdn.com/labreport4-131029210010-phpapp02/85/Chemistry-Lab-Report-on-standardization-of-acid-and-bases-4-320.jpg)



![Concentration of H2SO4 = No. of moles / (volume of diluted acid / 1000)
= 0.0309 mol / (25 / 1000) L
= 1.2 M
% Difference between Trail 1 and Trail 2 = [1.2 M / 1.2 M] * 100 %
= 100 %
Difference = (100 – 100) %
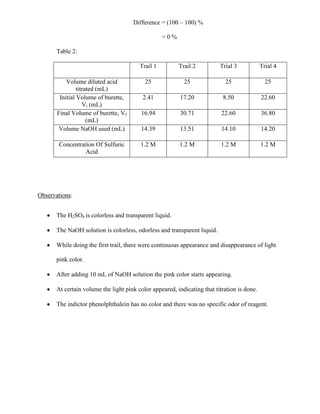
=0%
% Difference between Trail 1 and Trail 2 = [1.2 M / 1.2 M] * 100 %
= 100 %
Difference = (100 – 100) %
=0%
% Difference between Trail 1 and Trail 2 = [1.2 M / 1.2 M] * 100 %
= 100 %
Difference = (100 – 100) %
=0%
% Difference between Trail 1 and Trail 2 = [1.2 M / 1.2 M] * 100 %
= 100 %](https://image.slidesharecdn.com/labreport4-131029210010-phpapp02/85/Chemistry-Lab-Report-on-standardization-of-acid-and-bases-8-320.jpg)