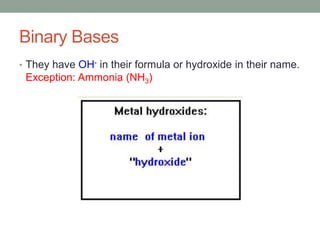
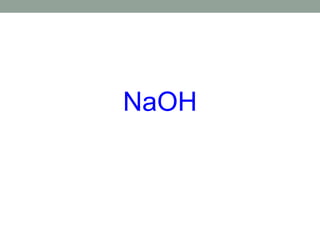
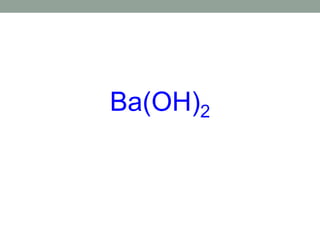
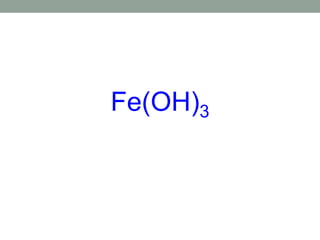
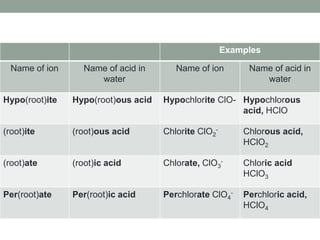
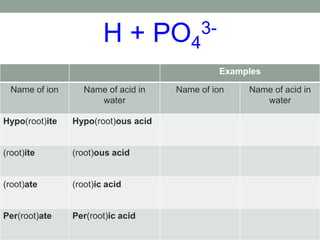
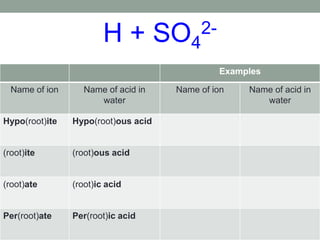
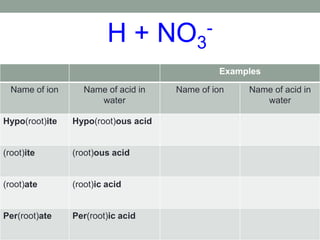
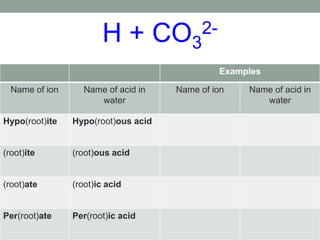
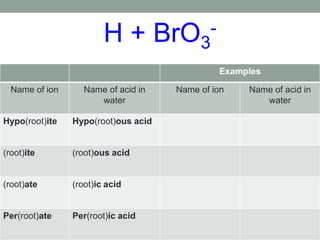
This document discusses nomenclature rules for binary compounds, binary bases and acids, and oxoacids. It explains that binary compounds contain two elements, binary bases contain OH- or have hydroxide in their name, and binary acids form when a hydrogen atom bonds to another element and dissolves in water. Rules are provided for naming binary acids based on the elements present. Oxoacids contain oxygen, hydrogen, and another element, and have suffixes that indicate the number of oxygen atoms such as -ous, -ic, and -ic. Examples are given for common oxoacid ions and their names.