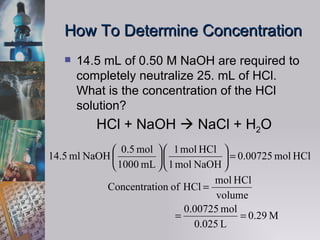
The document discusses acid-base titration, including the analyte, standard, neutralization reaction, equivalence point, and procedure. It explains that an acid-base titration involves titrating an acid with a base until the moles of H+ equal the moles of OH-. It also discusses using a pH sensor and indicator to determine the equivalence point, and using stoichiometry to calculate unknown concentrations from experimental data.