The document provides information about acid-base titrations including Bronsted-Lowry and Lewis acid-base theories, the self-ionization of water, and examples of water acting as an acid or base. It also discusses acid-base indicators and how they can be used to detect the equivalence point during titrations. Examples are given for titrations involving strong acid-strong base, weak acid-strong base, and weak base-strong acid. The dependence of the titration curve on concentration is illustrated and factors that can affect the choice of indicator are described.
![Bronsted-Lowry acid-base theory- An acid is a proton donor
and a base is a proton acceptor
The Bronsted-Lowry theory concentrates on proton
donation and acceptance.
•water molecules undergoing dissociation or self-ionisation
because of the reaction
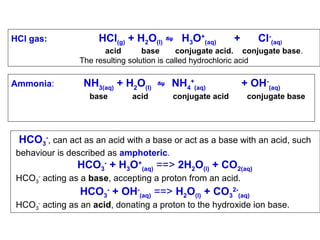
2H2O(l) ⇋ [H3O+(aq)] + [OH-(aq)]
BUT, in this reaction, water acts as both acid and base
Therefore water is an amphoteric oxide
•e.g. water acting as a base - proton acceptor with a stronger acid like
the hydrogen chloride gas
HCl(g) + H2O(l) ⇋ H3O+(aq) + Cl-(aq)
This is how hydrochloric acid is formed which you write simply as
HCl. e.g. water acting as an acid - proton donor with a weak BUT
stronger base like ammonia gas
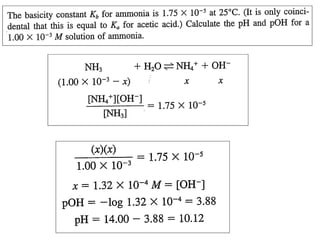
NH3(aq) + H2O(l) ⇋ NH4+(aq) + OH-(aq)
This is why ammonium solution is alkaline - sometimes wrongly called
'ammonium hydroxide' instead of aqueous ammonia](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-2-320.jpg)

![Equivalence point is determined by using an indicator (W.Ostald)
(pH-indicator)
HIn ⇋
H+ +
Acidic
Indicator(mol.form)
InOH
In-
HIn and In- are differently coloured
ionic form
⇋ In+ + OH-
InOH and In+ are differently coloured
Basic
ionic form
Indicator(mol.form)
⇋
Tautomeric transformation
HIn
Hin*
⇋ H+ + In-
HIn and In- are differently coloured
HIn ⇋ H+ + In[ H ][ In ]
kIn-a =
+
[ HIn]
[H ]
+
= kIn-a
pH = - logkIn-a
−
[ HIn]
[ In ] [ HIn]
−
- log [ In ]
−
pH = pKIn-a + log [ In ]
−
[ HIn]
Alkaline colour intensity
acidic colour intensity](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-6-320.jpg)
![[ In ]
When [ HIn] = 10, pH= pKIn-a + 1
[ In ]
and When [ HIn] = 1/10, pH= pK In-a - 1
−
−
Operationally,
pH= pKIn-a ± 1
[ In ] 10 times concn of HIn i.e., when the color due to [ In ] is dominant
−
−
During titration, if pH at equivalence point, lies in the range pK In-a ± 1,
that indicator can be used to detect the e.p. In the particular titration
InOH
⇋ In+ + OH-
[ In ][ OH ]
=
[ InOH ]
+
KIn-b
−
[ InOH ]
∴ [OH ] = kIn-b [ In ]
-
[ In ]
+
-log[OH ] or, pOH = pKIn-b + log10
-
[ InOH ]
[ In ]
pH = 14 - pKIn-b -log10 [ InOH ]
+
[ In ]
+
pH = pKIn-a -log10 [ InOH ]
+
pH + pOH =pKw=14
pH -14 = - pOH
HA ⇋ H+ + ApKa+pKb= pKw](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-7-320.jpg)
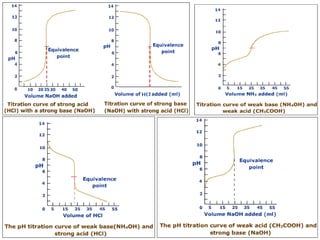
![1. Strong acid –strong base titrations
1(N) HCl ------- 1(N) NaOH
ml of
alkali
added
[H+] g-ion/L
pH
0
1
0
50
(50x 1/150)x 1
0.48
50 ml of 1N=150 ml of S(N), S= (50/150) (N)
99
(1/199)x 1
2.3
99.9
0.1/199.9
3.3
100
As in water
7
100.1
(0.1/200.1)
10.7
[OH-]= 0.1/200.1
101
p(OH)=3.3,
pH=10.7
110
[OH-]= 1/201
p(OH) = 2.3
[OH-]= 10/210
pH=11.7
12.7
150
[OH-]= 50/250
13.3
Volume of alkali added (NaOH)](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-9-320.jpg)

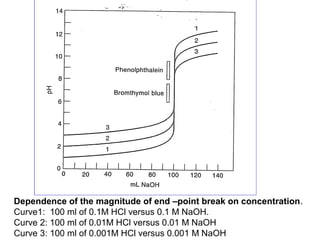
![2. Weak
acid - strong base titration
CH3COOH (HAc)
0.1 N, 100 ml
- NaOH
0.1N
ml of
alkali
added
[H+] g-ion/L
pH
0
√(1.75 x10-5 )x0.1
pH=4.8+log50/50
2.9
50
4.8(pKa)
HAc ⇋ H+ + Ac99
pH=4.8 +log 99/1
6.8
99.9
0.1/199.9
7.8
100
pH=7+2.4+½ log(0.01)
8.9
100.1
pH due to 0.1 ml NaOH 10.7
in 200.1ml soln
[OH-]= 0.1/200.1
101
p(OH)=3.3,
[OH-]= 1/201
p(OH) = 2.3
pH=10.7
pH=11.7
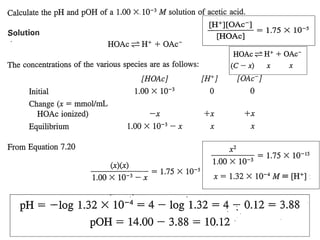
[ H ][ Ac ]
=
+
K
a
[ HAc]
−
=
+ 2
H
[ HAc]
Since [H+]= [Ac-] only for pure HAc
and [HAc] = c
∴[H+] = √(Ka . c)
(i) pH=½ pKa - ½log c
=½x4.8 -½log(0.1)=2.9](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-13-320.jpg)
![[ H ][ Ac ]
=
+
K
a
[ HAc]
−
[H ]
+
Ka[ HAc]
=
Ac -
[ ]
log Ac −
2. ∴pH = pK a +
[ HAc]
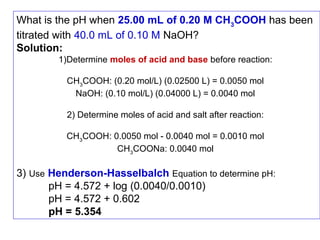
This form of ionisation constant equation is called the Henderson- Hasselbalch
equation. It is useful for calculating the pH of a weak acid soln containing its salt.
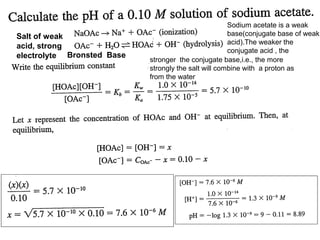
Salt of weak acid and strong base
2
HAc + OH- = Ac- + H2O
−
OH −
[ HAc] [ OH ]
K =
At equi.p. Ac undergoes hydrolysis
−
=
h
[ Ac ]
c
[HAc] = [ OH-]
Ac- + H2O ⇋HAc + OH2
OH − = K w / K a .c
[ HAc] OH − H + K
w
K =
∴OH − = Kw.C / Ka
x + =
h
−
Ac
K
H
a
1
1
1
[
]
∴ pOH = 2 pK w −
3.
pH =
1
1
1
pK w + pK a + log C
2
2
2
pK a − log c
2
1
1
∴ pH = pKw − 1 pK w − pK a − log c
2
2
2
2
3. General eqn for calculating e.p. When an weak acid is titrated with a strong
base and also when any salt of weak acid and strong base is dissolved in water](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-14-320.jpg)
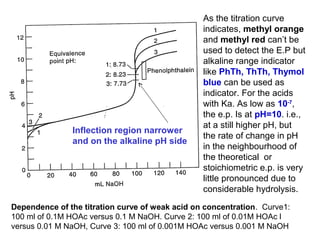
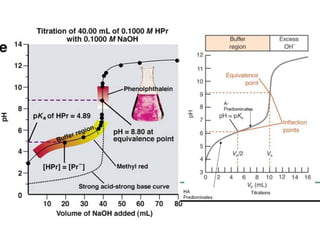
![3. Titration of Weak base with Strong acid
100 ml 0.1 N NH4OH vs.HCl(0.1N)
ml of acid [H+] g-ion/L
added
pH
0
14-2.37-0.5=11.1
11.1
50
14 - 4.8 – 0 = 9.2 (pKa of
conjugate acid of the
weak base)
9.2
99
14-4.8-log(99/1) = 7.2
7.2
99.9
14-4.8- log(99.9/1) = 6.2
6.2
100
7- 2.4 - ½log(0.05) = 5.25
5.25
100.1
S= (0.01/200.1)=3.8
3.8
NH4OH +HCl⇋ NH4Cl + H2O
NH4OH ⇋ NH4+ + OH-
[
Since [NH4+]= [OH-] only for free
base and [NH4OH] = c
∴OH − = Kb [ NH 4OH ]
pOH=½ pKb - ½log c
(i) pH=pKw -½ pKb + ½log c
0.1 mlx0.1N= S x 200.1 , S= 0.01/200.1
NH + OH −
4
K =
b
NH OH
4
Kb NH 4OH −
OH - =
NH 4+
∴
[
pH + pOH = pKw
pOH = pkw-pH
]
NH + OH −
−2
= OH
4
K =
[NH 4OH]
b
NH OH
4
]
pOH = pK b + log
∴pH = pK w − pK b − log
[NH4+ ]
[ NH4OH ]
[NH4+ ]
[ NH4OH ]](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-22-320.jpg)
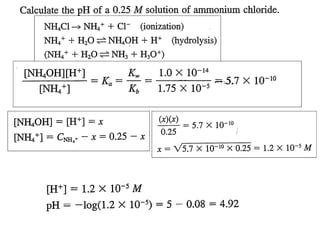
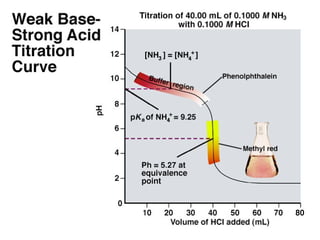
![At equivalent point only NH4Cl is present, but NH4+ undergoes hydrolysis(h)
NH4+ + H2O ⇋NH4OH + H+
K
h
=
H +
C
H +
X
NH +
H +
4
[ NH 4 OH ] H +
2
=
=
K
w
K
b
NH4Cl : a weak Bronsted acid will
follow hydrolysis
Kw
∴H + = K w .C / K b
K
b
1
1
1
pH = pK w − pK b − log C
2
2
2
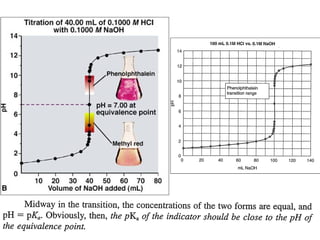
It is clear from the titration curve neither PhPh nor ThPh i.e., the alkaline
range indicator can be used to detect the e.p in the titration of 0.1N NH 4OH
agt a strong acid. Indicators with pH range on the slightly acidic side
such as M.O, M.R or BCG etc can be used for the purpose. BCG is
suitable for titration of all weak bases with Kb greater than10-6 .](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-23-320.jpg)
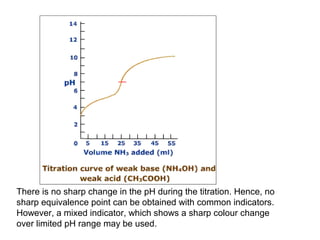
![4. Titration of weak acid - weak base
100 ml 0.1N
CH3COOH
VS.
NH4OH
(0.1N) aq NH3
At e.p. The pH will depend upon the relative strength of acid and base. In this
case both the weak acid anion and the weak base cation undergo hydrolysis.
Ac- + H2O ⇋ HAc + OH NH 4 + + H 2 O ⇋ NH 4 OH + H +
The hydrolytic equilibrium of a salt of weak acid and weak base is expressed by the eq.
[ΜΟΗ ][ΗΑ ]
+
Κ =
M + A +H2O ⇋ MOH +HA
h
Μ +Α − ....eqn 1
H +
[ΜΟΗ ][ΗΑ ] x x
Κ =
h
Μ + Α − H +
Kw
Kh =
....eqn 2
K a Kb
OH −
[ ΜΟΗ ][ ΗΑ ] x
OH −
Κh =
Μ + OH −
H + OH −
x
H +
A −
If x is the hydrolysis of 1 mol of the salt dissolved in V L of solution then
the individual concns are:
[MOH]= [HA]=x/V; [M+]= [A-] = (1-x)/V from eqn 1,
x x
V V
x2
=
Κh =
1 - x 1 - x
(1 - x) 2
V V
∴ Kh =
x
(1 - x)
....eqn 3](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-25-320.jpg)
![HA ⇋ H+ + A-
Ka
H + A −
=
[ HA ]
∴ H + = K a .
∴ H + = K a [ HA ] = K a ( x ) = Ka . K
A -
h
(1 − x)
Kw
=
K a Kb
K a .K w
Kb
No conc. term for the salt
produced appears
If the ionisation constant of the acid and the base
1
1
1
pH = pK w + pK a − pK b are equal, that is Ka=Kb, pH= ½pKw =7.0,
2
2
2
pH of Ammonium acetate is 7.0+2.7 - 2.7 = 7.0
Hence, during titration of weak acid agt a weak base pH at e.p. is independent of
the concn. of acid and base and depends on only the relative strength of acid
and base.
Accordingly, no sharp colour change obtd. at e.p with any simple pH
indicator. However, a mixed indicator of suitable composition which exhibits
a sharp colour change over a very limited pH range may sometimes be used for
the detection of e.p.Thus, HAc-NH4OH titration, neutral red + methylene blue
has been used with some success.
On the whole it is better to avoid such titrations by indicator method. Usually
potentiometric and conductometric titrations are preferable in such cases.](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-26-320.jpg)
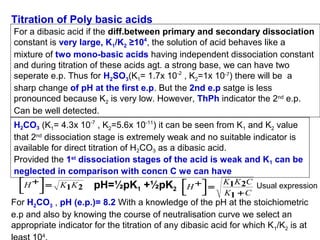
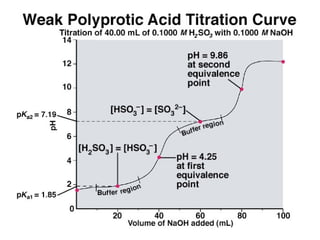
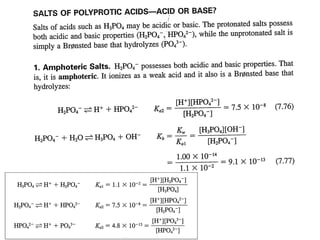
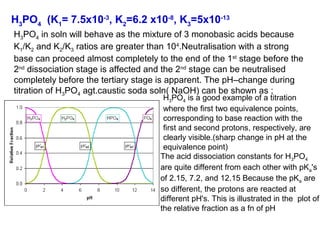
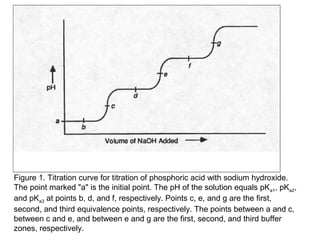
![(i)With 50% of 1st acidity (1st replaceable H+ half neutralised)
[H PO ]
−
pH = pK1 +log10
(ii) At 1st e.p.
2
4
[ H 3PO 4 ]
= pK1 = 2.1
pH = ½pK1 + ½pK2 = ½(2.1+7.2)= 4.65
(iii) With 1.5 equivalence of alkali added,
pH = pK2 +log10
[HPO ]
[H PO ]
4
2
−2
4
−
= pK2 = 7.2
(iv) With 2nd equivalence point,
pH = ½pK2 + ½pK3 = ½(7.2+12.3)= 9.75
(v) With 2.5 equivalence of alkali added,
pH = pK3 +log10
(vi) With 3rd equivalence point,
PO − 3
4
HPO − 2
4
= pK1 = 2.1
pH = ½pKw + ½pK3 +½log C = ½(7+6.5-0.5)= 12.65](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-33-320.jpg)
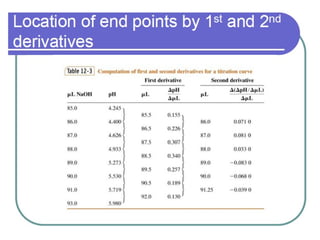
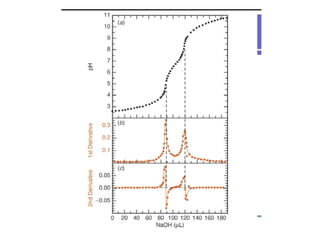
![The pH at points where the relative fraction of two species are equal, e.g.,
where two relative concentration lines cross, have a simple relationship to
the acid equilibration constants. For example, the first crossing occurs for
[H3PO4] = [ H2PO4-]. The relationship to pH is most easily found by
recognizing that all principle species are given in the first proton ionization
equation](https://image.slidesharecdn.com/titrimetreyasanalyticaltool-140120005800-phpapp02/85/Titrimetrey-as-analytical-tool-P-K-MANI-34-320.jpg)