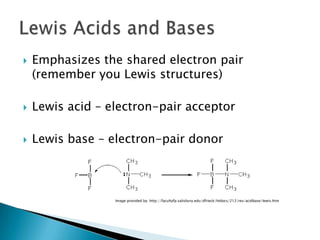
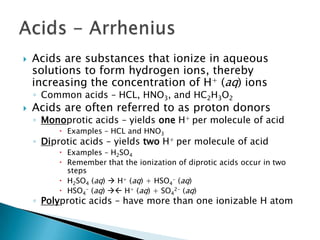
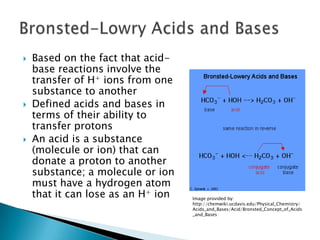
Acids are substances that donate protons (H+) in aqueous solutions, increasing the concentration of H+ ions. Common acids include HCl, HNO3, and HC2H3O2. Bases are substances that accept H+ ions, producing hydroxide (OH-) ions when dissolved in water. Common bases include NaOH, KOH, and Ca(OH)2. Strong acids and bases are completely ionized in solution, while weak acids and bases are only partially ionized. The strength of an acid or base determines how reactive it is.




![Because the molar concentration of H+ (aq) in an aqueous solution is usually very small, we usually express [H+] in terms of pH, which is the negative logarithm in base 10 of [H+]pH = -log[H+] A solution is neutral if [H+] = [OH-]Don’t forget your constants that can help calculate pH and concentrationsKw (ion-product) for water = 1.0 x 10-14 (at 25oC) Ka (acid-dissociation) – the larger the Ka value, the stronger the acidKb (base-dissociation) – refers the equilibrium in which a base reacts with water to form the corresponding conjugate acid and OH-The product of the acid-dissociation constant for an acid and the base-dissociation constant for its conjugate base is the ion-product constant for water (Ka x Kb = Kw)Measuring pH:pH meter, acid-base indicators – colored substance that itself can exist in either an acid or a base form; have different colors, indicator turns one color in an acid and another color in a baseThe pH Scale](https://image.slidesharecdn.com/acidsandbases-110723110452-phpapp02/85/Acids-and-Bases-8-320.jpg)