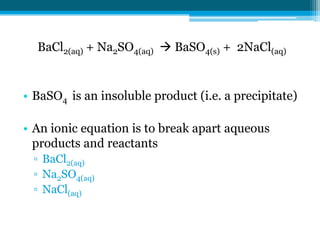
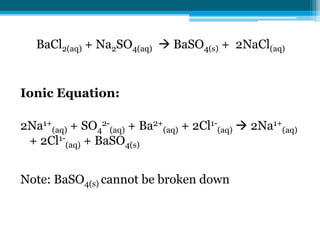
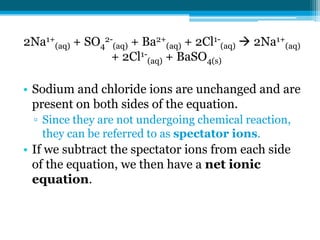
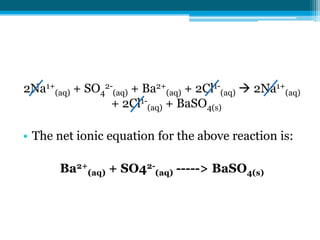
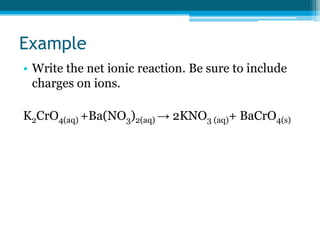
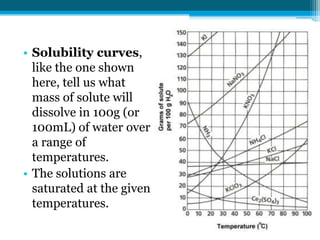
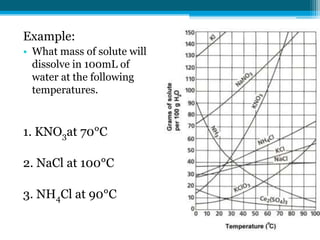
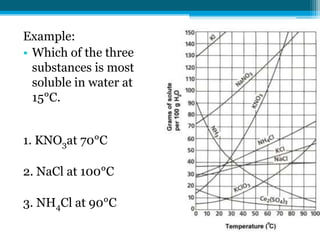
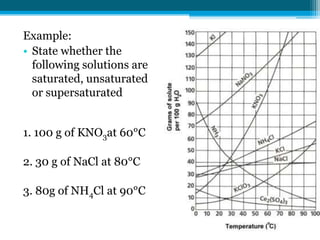
This document discusses net ionic equations and solubility curves. It provides examples of writing net ionic equations by identifying spectator ions and writing the net reaction. It also discusses solubility curves and using them to determine the solubility of substances at different temperatures and whether solutions are saturated, unsaturated or supersaturated. Homework includes practice with net ionic equations and using solubility curves to solve problems involving precipitation reactions and limiting reactants.