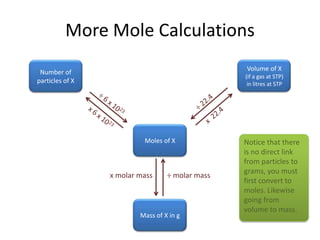
The document defines key concepts related to the mole including:
- The mole is a unit that represents 6.022x10^23 particles and allows chemists to conveniently work with substances at a macroscopic scale.
- One mole of any substance has a mass in grams numerically equal to its molar mass.
- Common calculations involve determining moles, mass, particles, or volume of a substance using its molar mass and amount in moles.
- The molar volume of a gas is 22.4L at STP allowing for volume calculations.


![(i)
(ii)
How many molecules are there in 0.5 mols of chlorine gas?
How many atoms are there in 2 mols of water?
(iii)
How many electrons are there in 1.5 mols of Calcium?
(i)
1 mol of Cl2
6 1023 molecules
0.5 mols of Cl2
(ii)
0.5 6 1023
1 mol of H2 0 6 1023 molecules
[Cl2 is composed of molecules]
3 1023 molecules
[H2 O is composed of molecules]
2mols of H2 0 2 6 1023 1.2 1024 molecules
Each molecule of water contains 3 atoms
1.2 1024 molecules contains 3 1.2 1024
(ii)
3.6 1024 atoms.
1 mol of Ca 6 1023 atoms [Ca is composed of atoms]
1.5 mols of H2 0 1.5 6 1023
9 1023 atoms
Each atom of Calcium contains 20 electrons
9 1023 atoms contain 23 9 1024
2.07 1025 electrons.](https://image.slidesharecdn.com/3-140309061656-phpapp02/85/3-3-the-mole-4-320.jpg)


![(i)
How many moles are there in 990 g of cabon dioxide (CO2 )
(ii)
The daily intake of calcium for an adult is 800 mg, how many moles of calcium is this?
(i)
1 mol of CO2
44 g
1
mols of CO2 1g
44
1
990 mols of CO2 990 g
44
22.5 mols of CO2 990 g
(ii)
1 mol of Ca 40 g
1
mols of Ca 1g
40
1
800 10 3 mols of Ca 800 10 3 g
40
0.02 mols of Ca 800 10 3 g
[800mg 800 10 3 g]](https://image.slidesharecdn.com/3-140309061656-phpapp02/85/3-3-the-mole-10-320.jpg)

![The Nissan Micra 1.5 Diesel SVE is quoted to have a CO2 emission figure of 120 g / km.
For a 40 km round trip to work calculate:
(i)
(ii)
(iii)
the mass of CO2 produced.
the number of moles of CO2 produced.
the volume of CO2 produced at room temperature and pressure.
[Molar volume at room temperature and pressure 24.0 litres]
(i)
120 40 4800 km
(ii)
no. of mols
(iii)
1mol of CO2
actual mass
molecular mass
4800
no. of mols
mols
44
no. of mols 109.09 mols
24.0 L
109.09 mols of CO2
109.09 mols of CO2
109.09 24L
2594.16 L](https://image.slidesharecdn.com/3-140309061656-phpapp02/85/3-3-the-mole-13-320.jpg)
![How many iron atoms should be consumed daily to meet the recommended daily intake
of iron in the diet of 0.014 g?
no. of mols
acutalmass
molecular mass
0.014
56
no. of mols 2.5 10 4 mols
no. of mols
1mol 6 10 3 atoms
2.5 10 4 mols 2.5 10
4
2.5 10 4 mols 1.5 10
0
6 10 3 atoms
atoms
[H2007, Q4 (e)]](https://image.slidesharecdn.com/3-140309061656-phpapp02/85/3-3-the-mole-14-320.jpg)