This document provides information about the mole as a unit of measurement in chemistry. It defines the mole as a preferred unit used to represent small numbers of particles, such as atoms or molecules. The key points are:
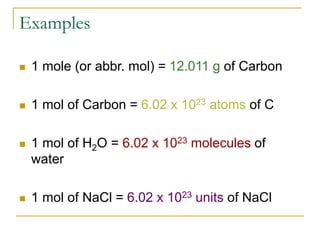
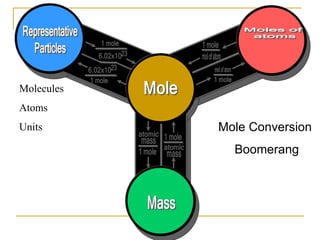
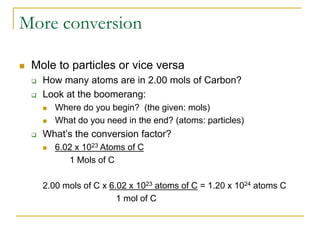
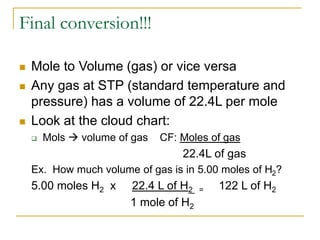
- 1 mole is equal to 6.022 x 1023 representative particles, whether atoms, molecules, formula units, etc.
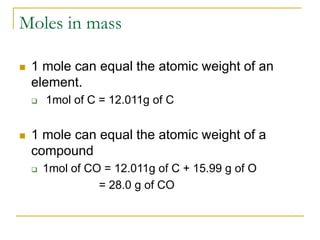
- Common mole-particle conversions include 1 mole of carbon = 12.011 g of carbon and 1 mole of any substance containing 6.022 x 1023 representative particles.
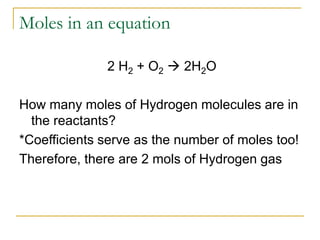
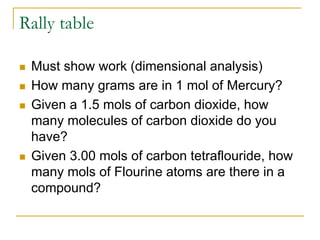
- Mole conversions can be used to determine the number of moles, grams, or particles between different units using dimensional analysis and provided conversion factors.