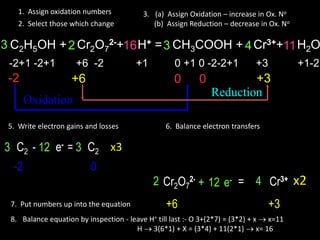
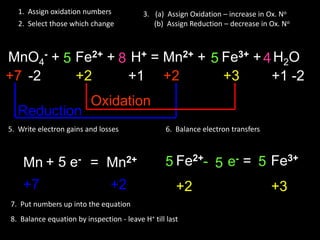
The document provides steps for balancing chemical equations using oxidation numbers:
1. Write out reactants and products and assign oxidation numbers.
2. Identify atoms whose oxidation numbers change during oxidation and reduction reactions.
3. Write the electron transfers involved in oxidation and reduction.
4. Balance the overall electron transfers between reactants and products.
5. Insert the balanced electron transfer numbers into the chemical equation.
6. Balance the overall chemical equation by inspection, balancing hydrogen last.


![Sequence of events
• Write out the formulae of all reactants and
products
• Assign oxidation numbers to all atoms
• Pick out the atoms whose oxidation numbers
change
• Write down the electron movements
• Balance the electron movements
• Enter the numbers from balanced electron
movement into the overall equation
• Balance equation by inspection - [do H last]](https://image.slidesharecdn.com/2-140309055206-phpapp02/85/2-6-2-balancing-equation_using_oxidation_numbers-3-320.jpg)
![Sequence of events
•
•
•
•
•
•
•
Write down reactants and products
Assign oxidation numbers
Identify oxidation and reduction
Write down changes and electrons involves
Balance e- change
Put changes into equation
Balance equation by inspection [H last]](https://image.slidesharecdn.com/2-140309055206-phpapp02/85/2-6-2-balancing-equation_using_oxidation_numbers-5-320.jpg)