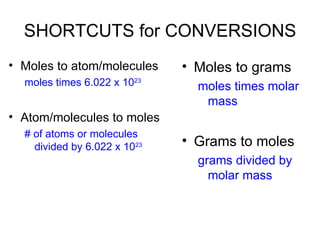
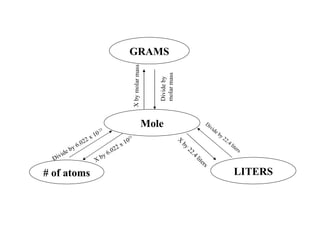
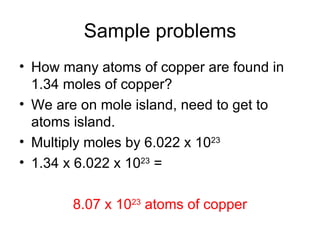
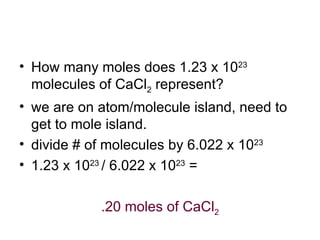
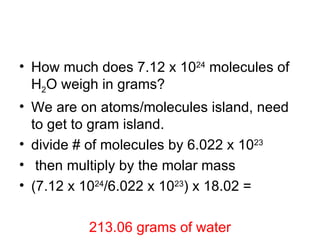
The document discusses mole conversions between number of atoms/molecules, moles, and grams. It provides shortcuts for common conversions between these units and works through sample problems converting between atoms, molecules, moles, and grams of various substances using their molar masses.