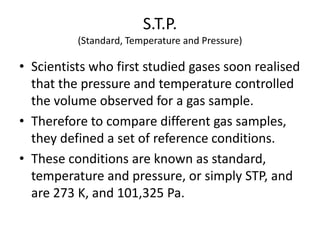
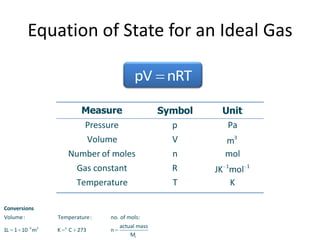
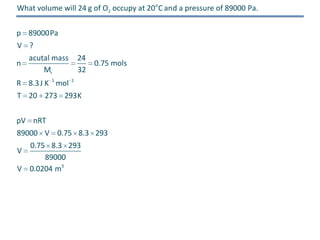
The document discusses several gas laws:
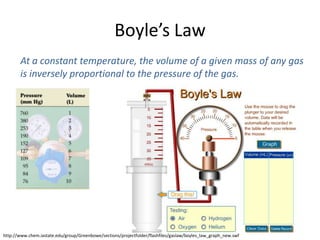
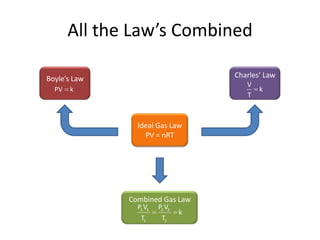
- Boyle's law states that at constant temperature, the volume of a gas is inversely proportional to its pressure.
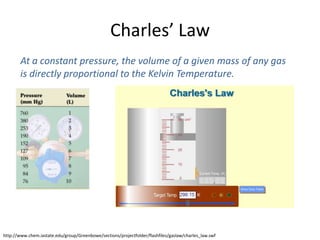
- Charles' law states that at constant pressure, the volume of a gas is directly proportional to its kelvin temperature.
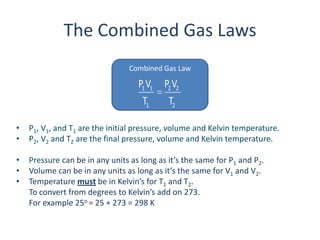
- Combined gas law allows calculation of gas properties when temperature and/or pressure change.
It also discusses concepts like the kinetic molecular theory of gases and how real gases deviate from ideal gas behavior due to intermolecular forces and molecular size. Sample calculations demonstrate use of the gas laws and kinetic theory.