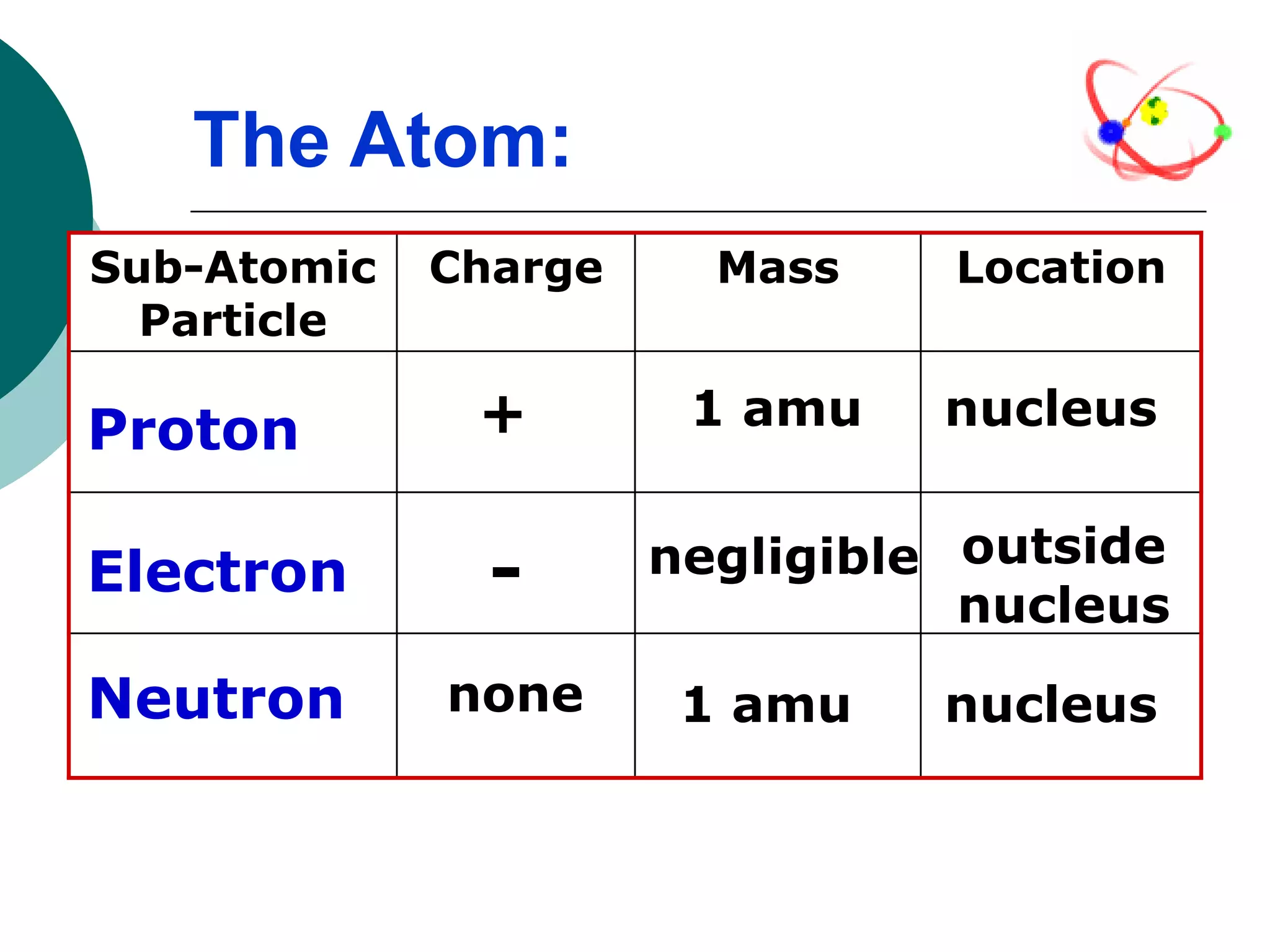
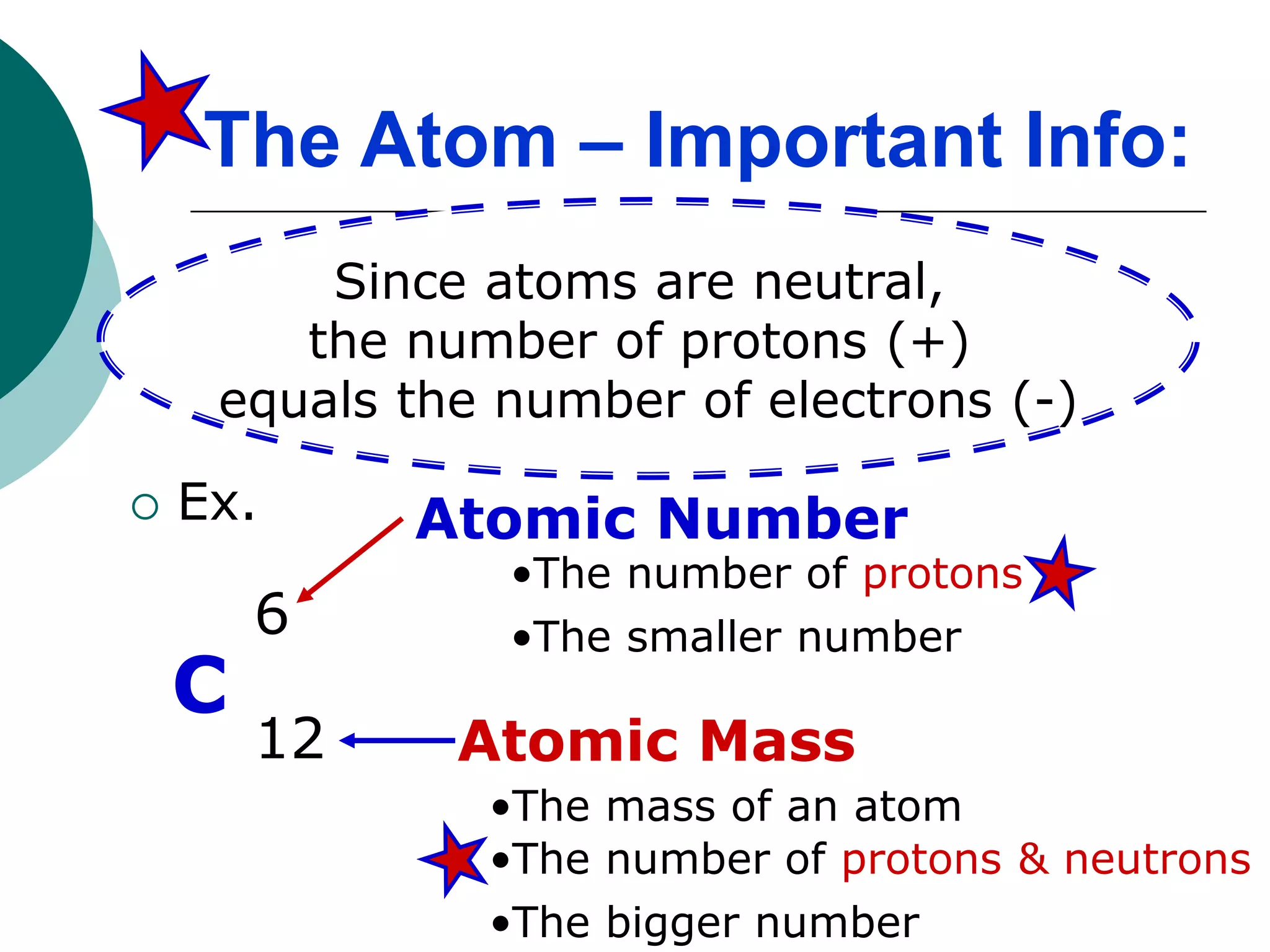
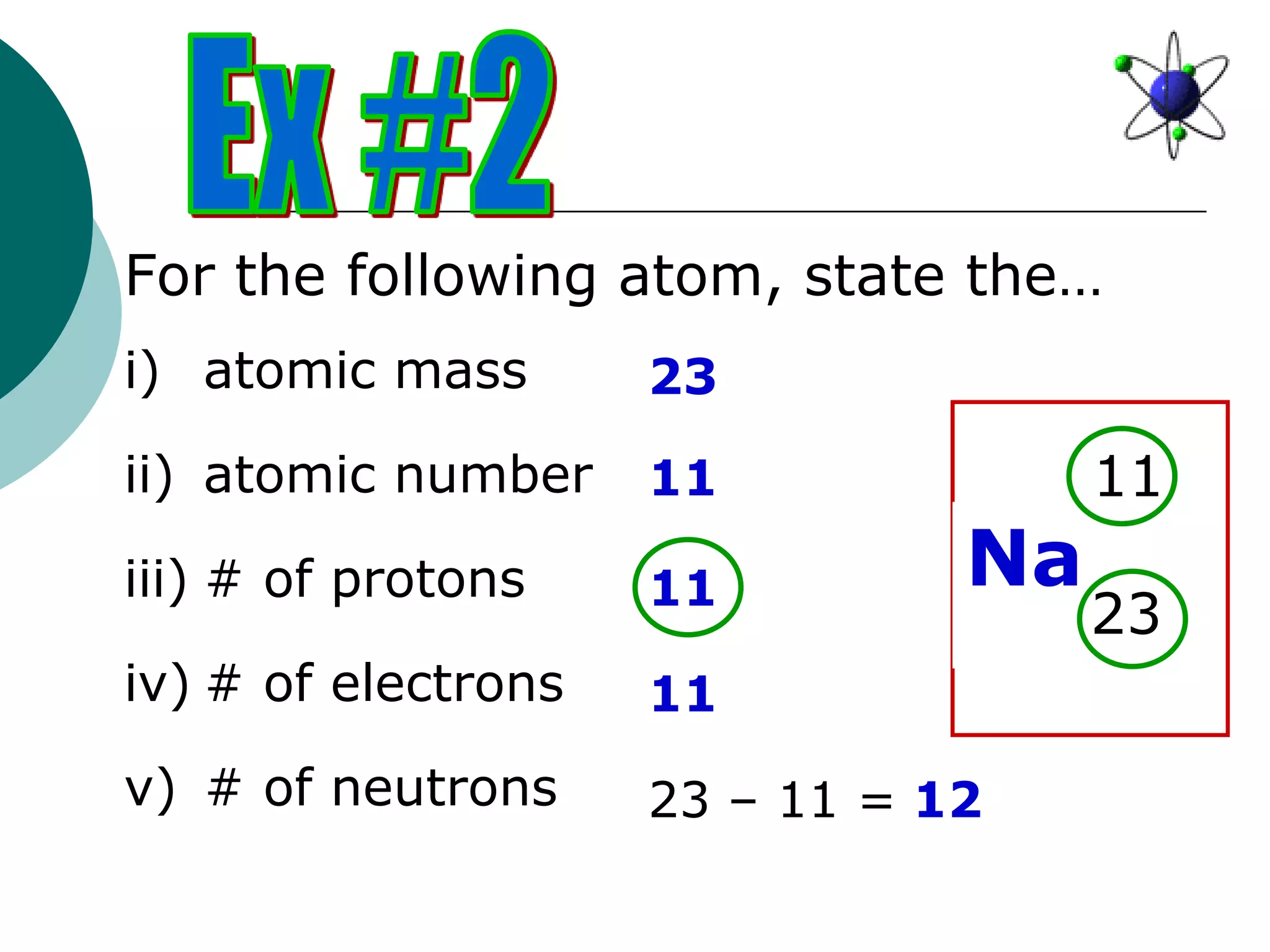
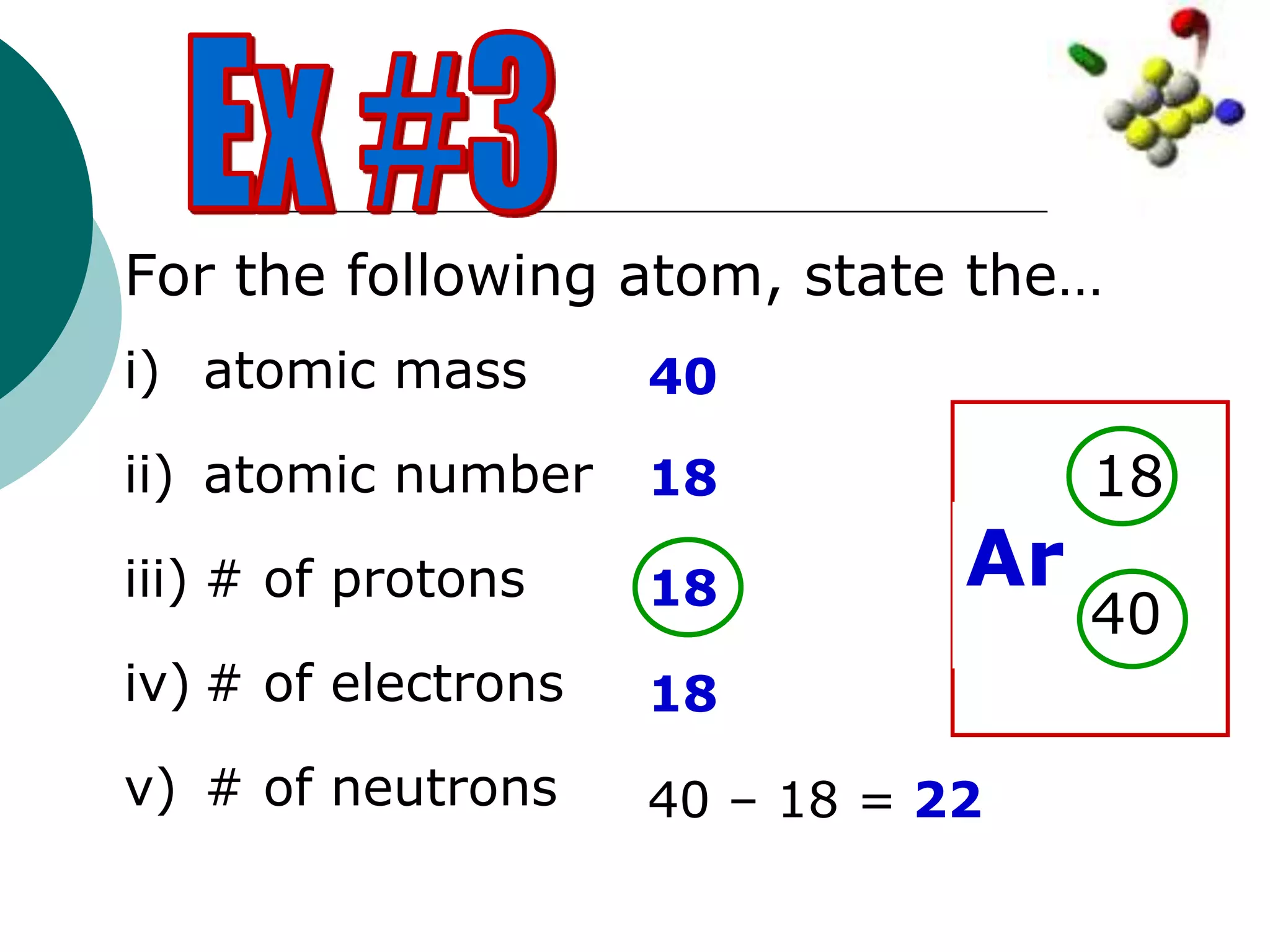
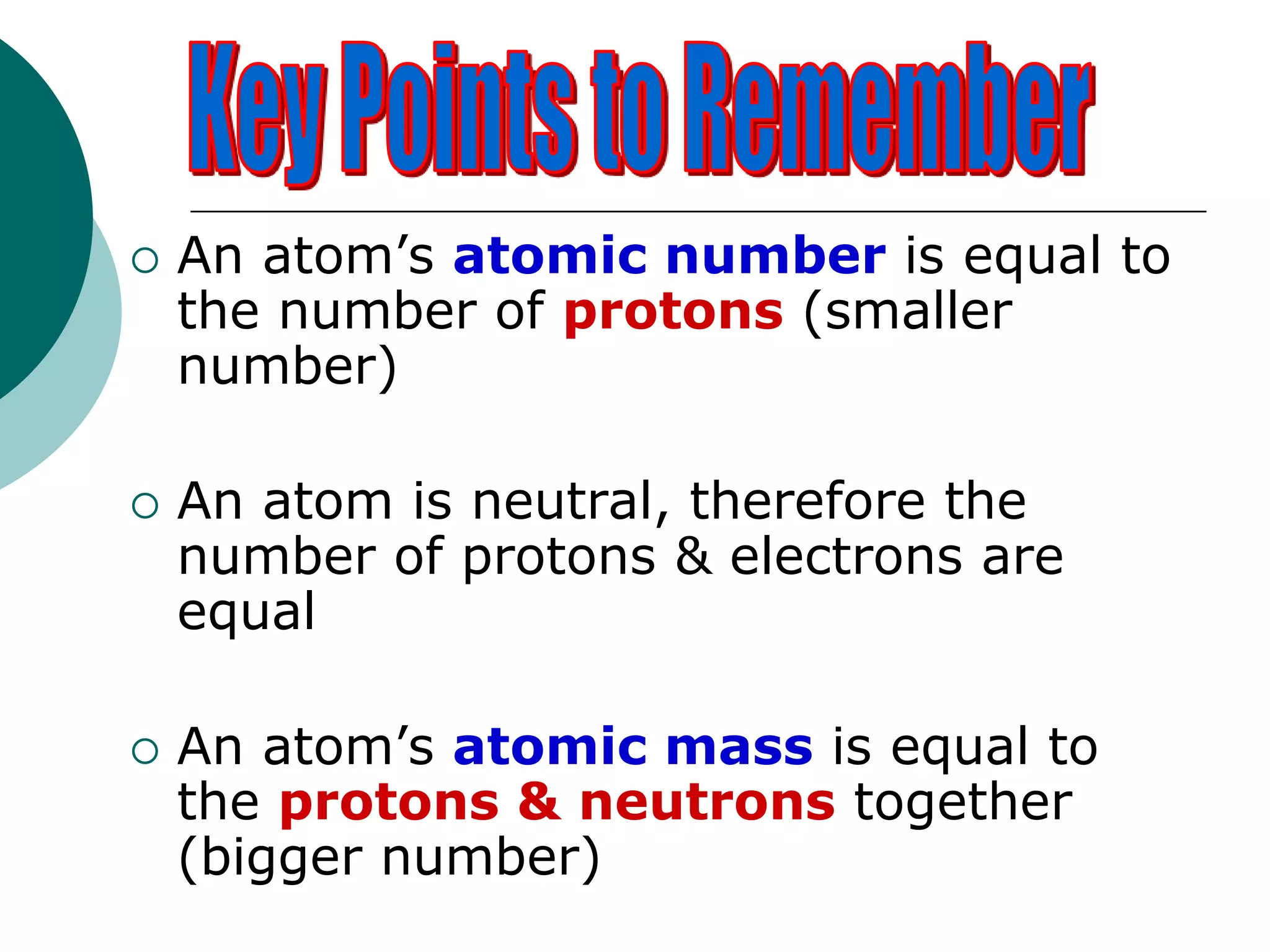
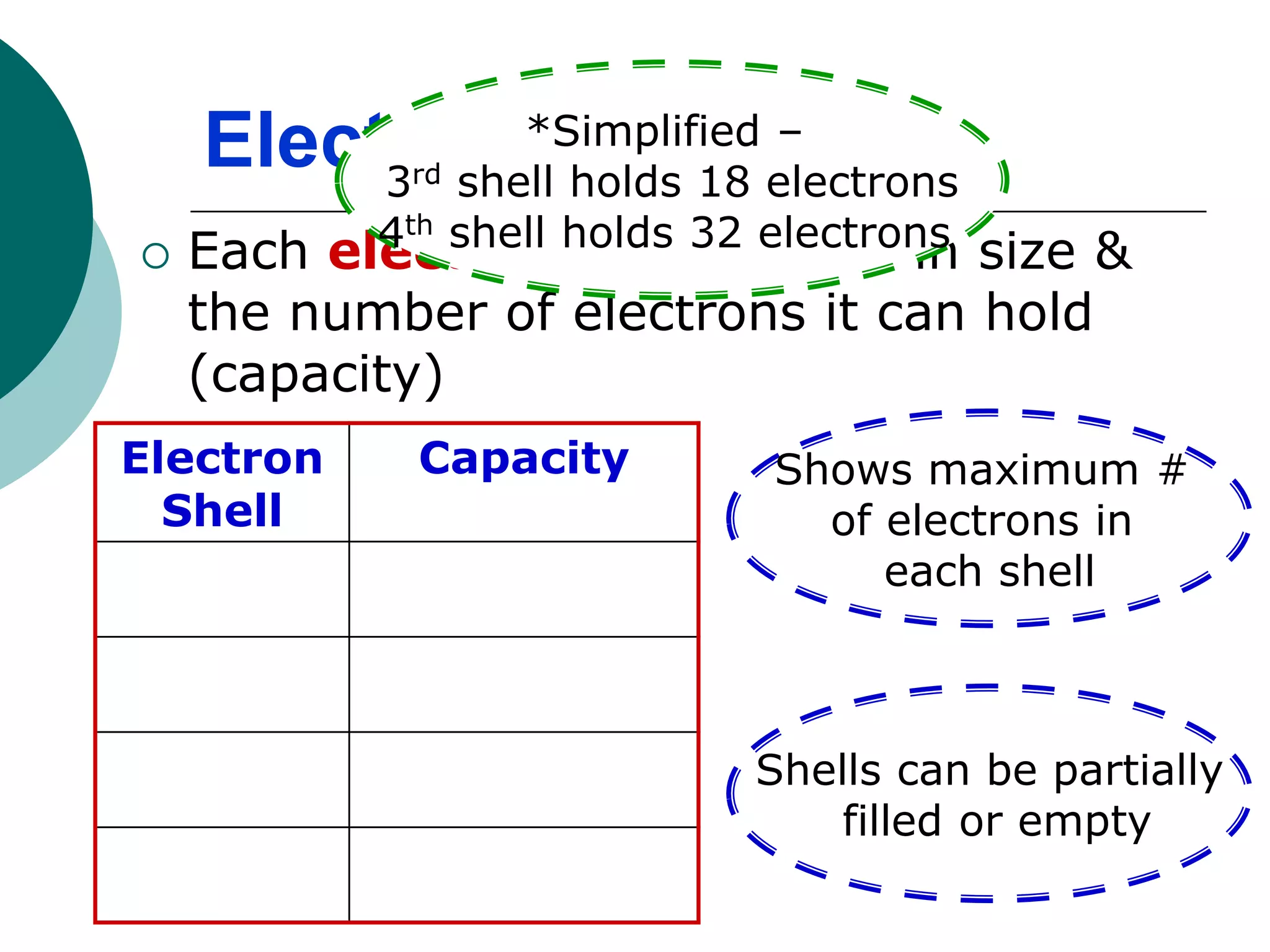
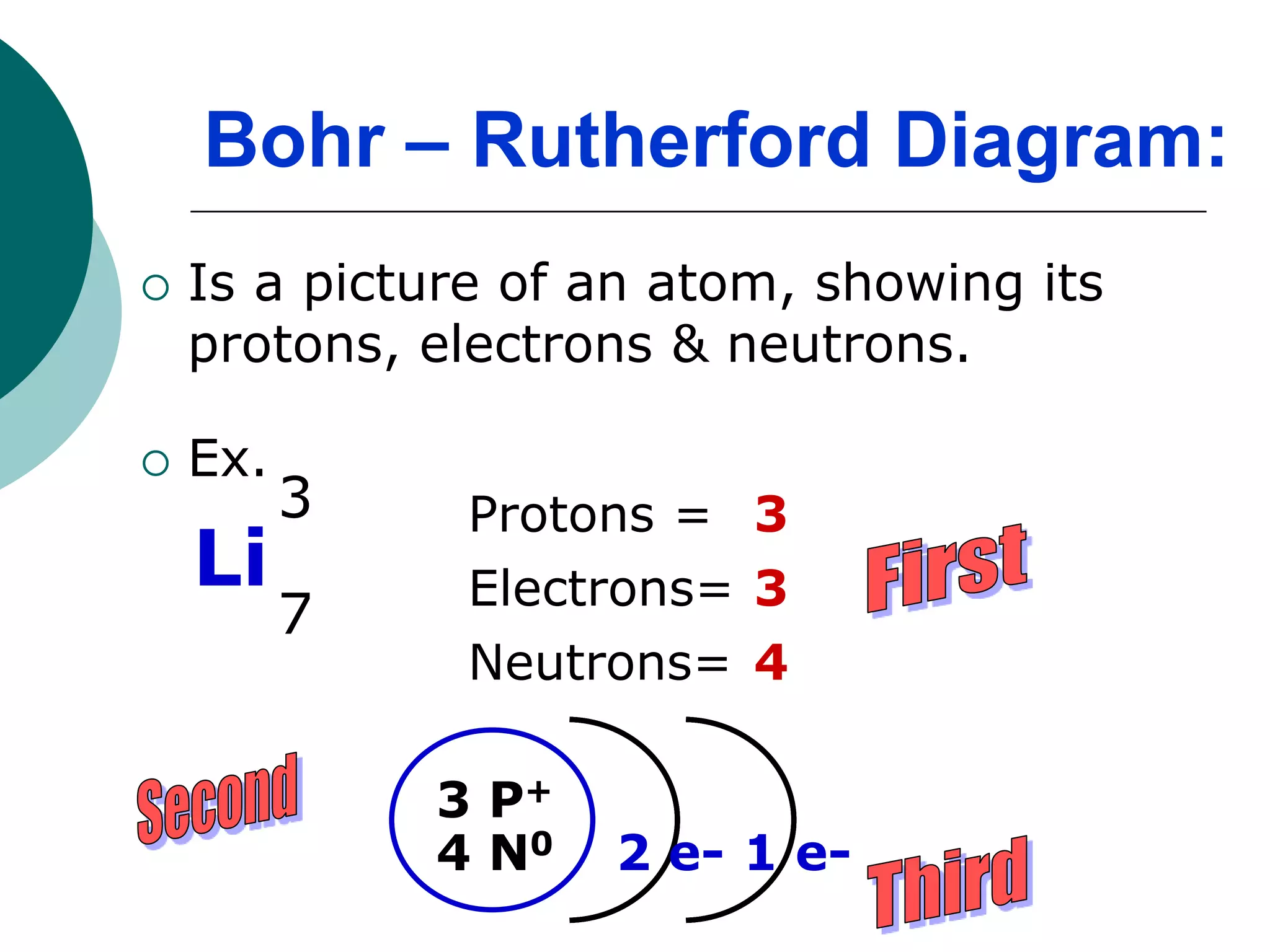
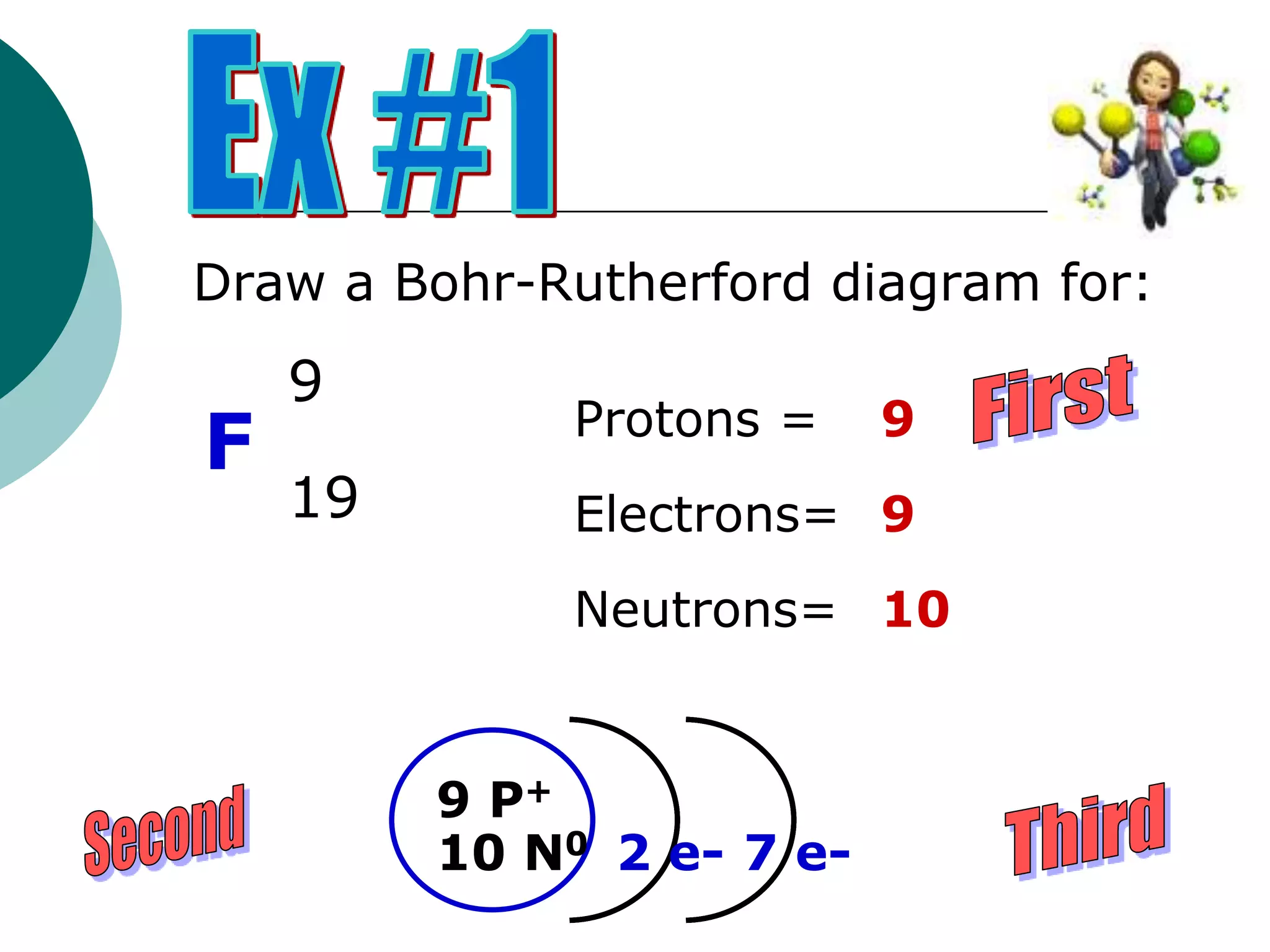
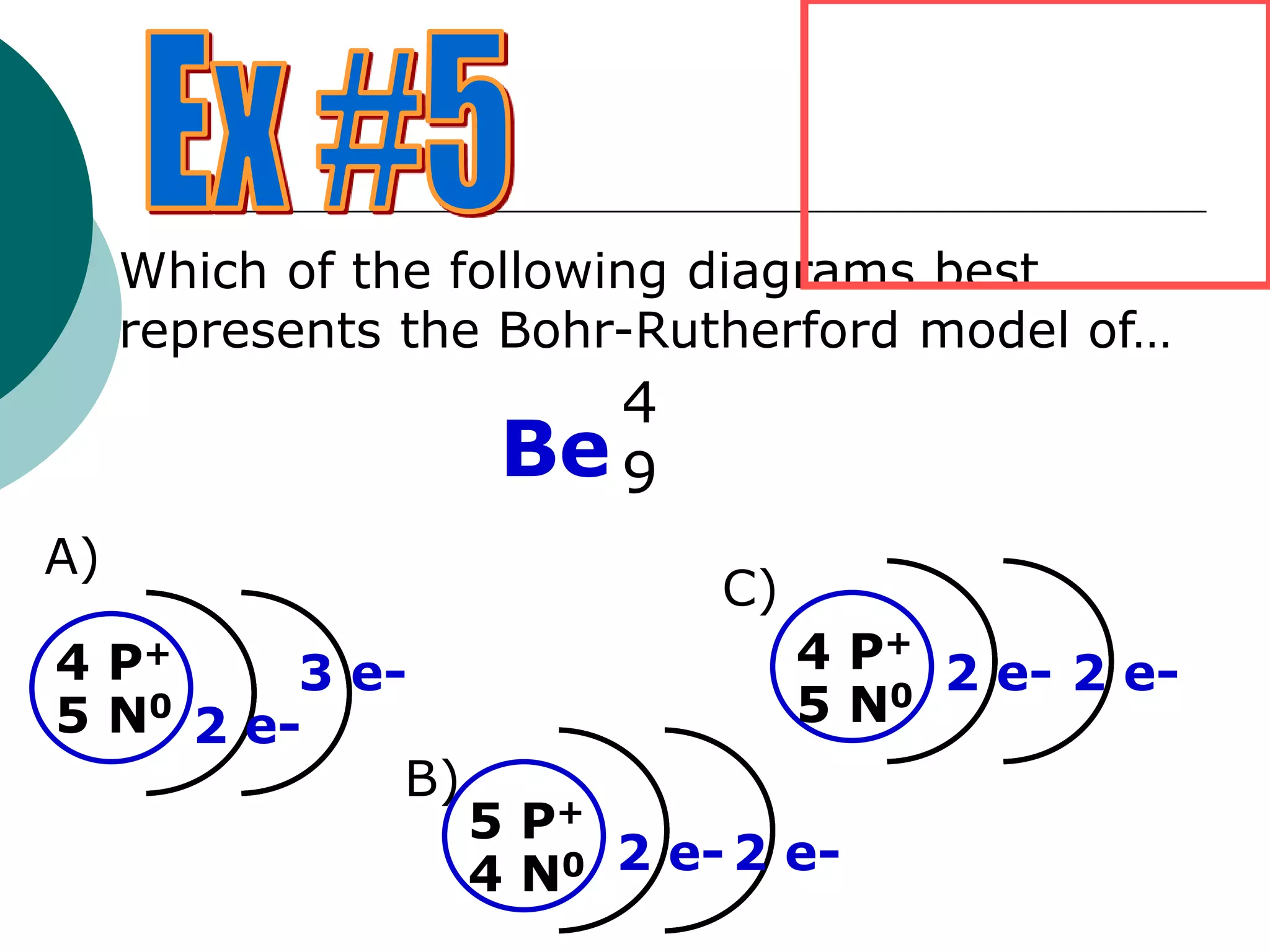
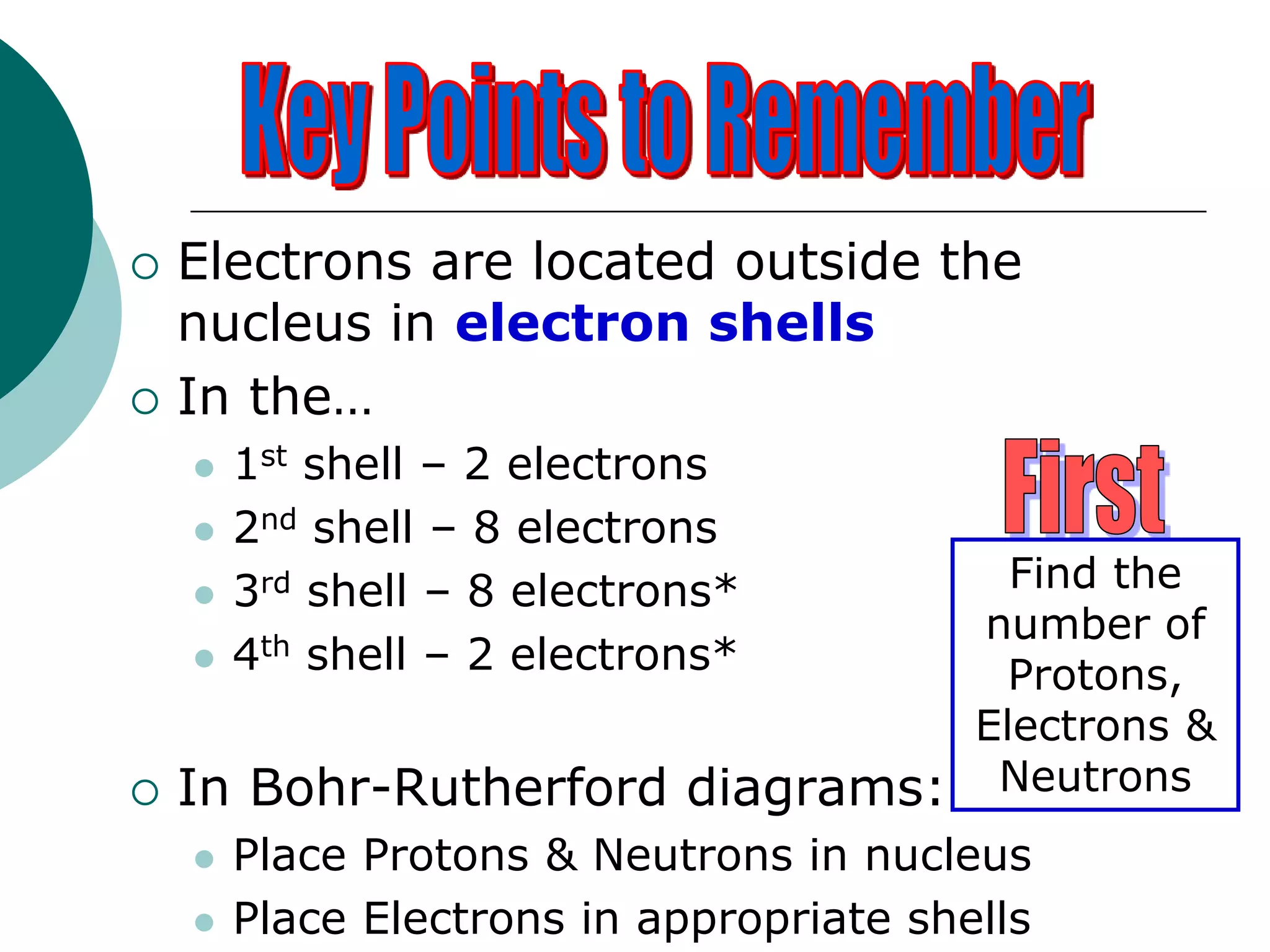
The document describes the Bohr-Rutherford atomic model. It states that the atom consists of a small, dense nucleus containing protons and neutrons, surrounded by electrons in shells. The atomic number equals the number of protons, and a neutral atom has the same number of protons and electrons. Electrons are located in shells outside the nucleus, with the first shell holding 2 electrons and subsequent shells able to hold more. Diagrams illustrate atoms showing their arrangement of subatomic particles. Examples are provided of looking up atomic properties and drawing Bohr-Rutherford diagrams for different elements.