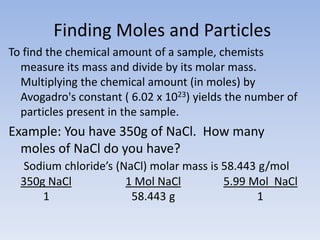
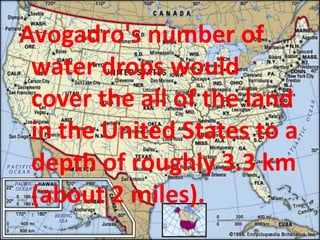
The mole is a unit used in chemistry to measure the amount of substance. It represents 6.022x10^23 elementary entities which could be atoms, molecules, ions, etc. The mass of one mole of a substance in grams is equal to its molar mass in g/mol. To calculate the number of moles or particles in a sample, its mass is divided by the molar mass of the substance. While difficult to visualize, one mole contains an immense number of particles on the scale of Avogadro's number.