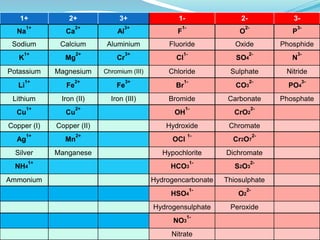
This document provides a table and instructions for writing chemical formulas using electrovalencies. The table lists common ions in the columns for positive and negative charges. The document explains that metals are positively charged while non-metals are negatively charged. It provides examples of writing formulas such as NaCl, CaCl2, MgO, and Al(OH)3 by matching the charges of ions and adding subscripts. Key ions like sulfates, hydroxides, and carbonates are identified that are essential to know for writing chemical formulas.

![ This is a simple way to learn how to write
chemical formulae. There are other ways to
work them out but this is simple although it does
involve some learning by heart
On a graph page at the back of your practical
notebooks draw lines on all the red [or heavier]
lines.
Head the columns +1, +2, +3, blank, -1, -2, -3.
Enter the appropriate radical(ion) and its charge
in each square as shown in the next slide](https://image.slidesharecdn.com/2-140309055105-phpapp01/85/2-1-1-compounds-simple_chemical_formulae-2-320.jpg)


![ Positive ion [metal] goes first
Negative ion [non-metal] or radical goes
second
Multiply each ion [radical] by the smallest
possible integer to make the total of + and
– the same
Place this number as a subscript after each
ion. If the ion is complex [i.e. a radical] put
it in brackets and put the subscript outside
the brackets
P J Jackson
6](https://image.slidesharecdn.com/2-140309055105-phpapp01/85/2-1-1-compounds-simple_chemical_formulae-6-320.jpg)








![Chromium carbonate
Cr3+ and CO32Three pluses and two minus
So two Cr3+ [6+]are needed to cancel out
the 3 CO32- [6-]
Therefore Cr2(CO3)3
Notice that as before the CO3 is put in
brackets as it is a compound ion](https://image.slidesharecdn.com/2-140309055105-phpapp01/85/2-1-1-compounds-simple_chemical_formulae-15-320.jpg)

![Essential to know
Sulphates [SO42- ]
Sulphites [SO32-]
Hydroxides [OH-1]
Carbonates [CO32-]
Hydrogencarbonates [HCO31-]
Nitrates [NO31-]
Of the first 36 elements – excluding the d-
block elements – where they exist
P J Jackson
17](https://image.slidesharecdn.com/2-140309055105-phpapp01/85/2-1-1-compounds-simple_chemical_formulae-17-320.jpg)
![Positive ions
Lost electrons
One positive charge for each electron lost
If doing s,p,d,f . electron pattern then put
in square brackets with charge outside
The pattern inside the bracket will be of
the nearest Noble gas [He, Ne or Ar]
Only required for first 20 elements
P J Jackson
18](https://image.slidesharecdn.com/2-140309055105-phpapp01/85/2-1-1-compounds-simple_chemical_formulae-18-320.jpg)
![Negative Ions
Gain electrons
One negative charge for each electron
gained
If doing s,p,d,f . electron pattern the
put in square brackets with charge
outside
The pattern inside the bracket will be
of the nearest Noble gas [He, Ne or Ar]
Only required for first 20 elements
P J Jackson
19](https://image.slidesharecdn.com/2-140309055105-phpapp01/85/2-1-1-compounds-simple_chemical_formulae-19-320.jpg)
