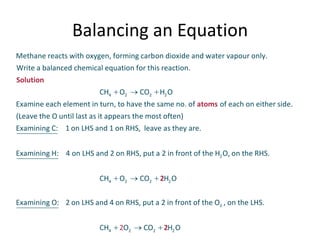
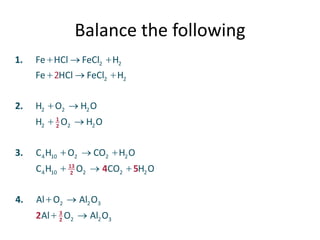
Chemical equations are used to represent chemical changes. They show the reactants and products of a reaction. A balanced chemical equation has the same number and type of atoms of each element on both sides. To balance an equation, the coefficients of the molecules are adjusted without changing subscripts so that atomic balance is achieved. Balanced equations indicate the mole ratios in which substances react, allowing for calculations relating amounts in moles of reactants and products.