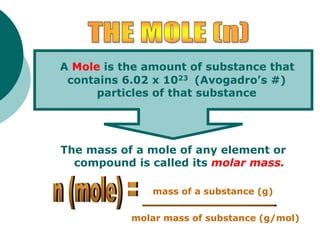
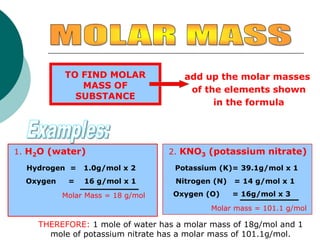
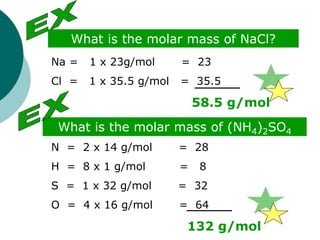
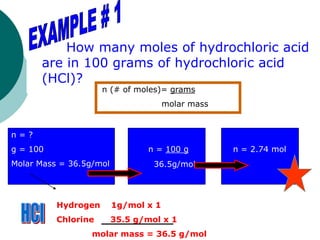
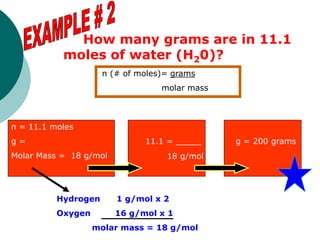
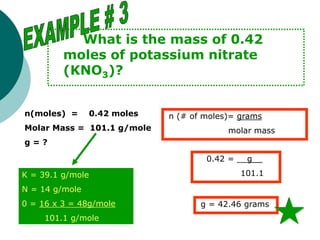
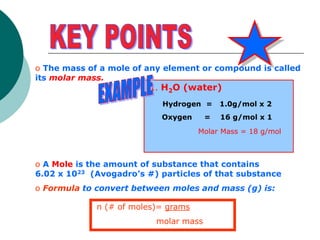
This document provides information about moles, molar mass, and stoichiometry calculations. It defines a mole as the amount of substance containing 6.02 x 1023 particles and molar mass as the mass of one mole of a substance. Formulas are given for calculating molar mass from elemental compositions and for converting between mass and moles using molar mass. Examples show calculations of molar mass for compounds and conversions between grams and moles for different substances.