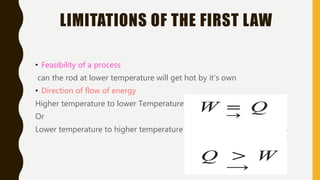
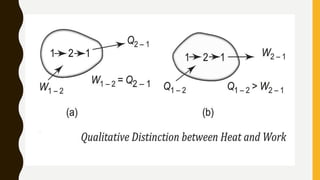
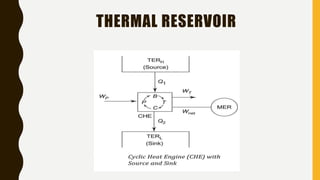
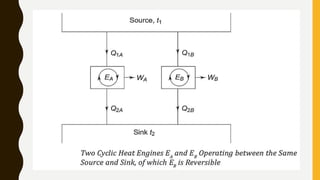
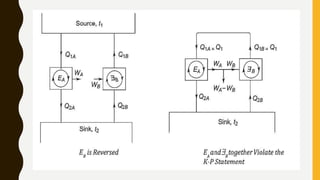
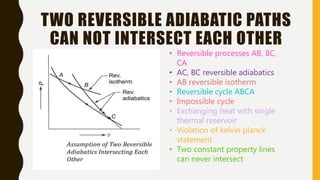
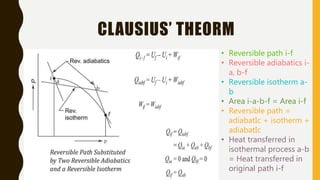
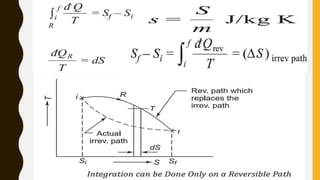
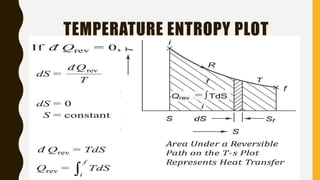
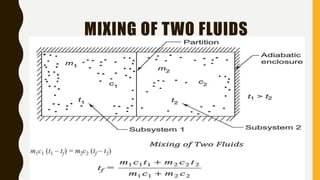
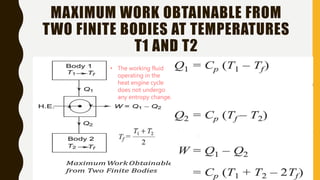
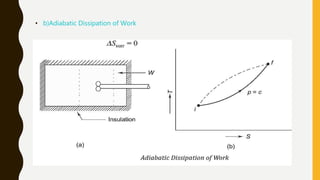
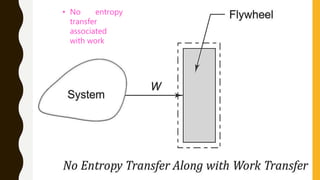
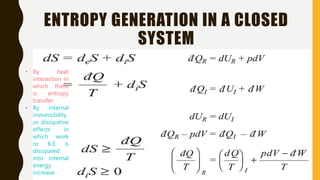
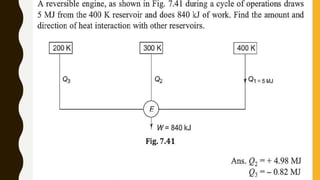
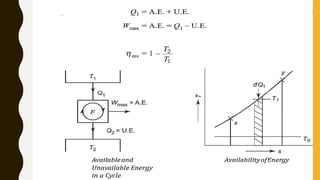
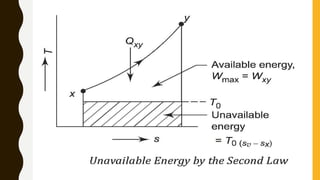
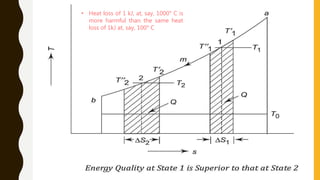
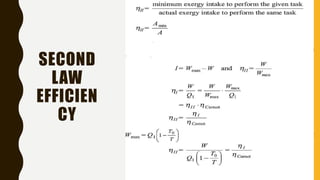
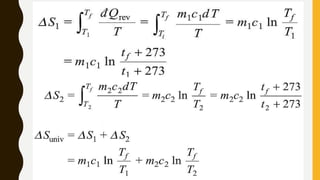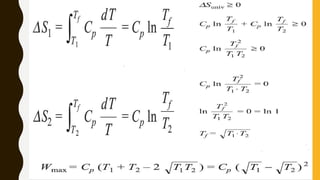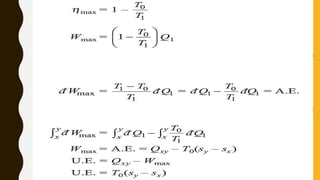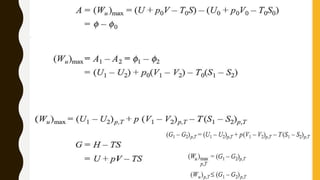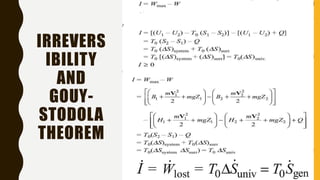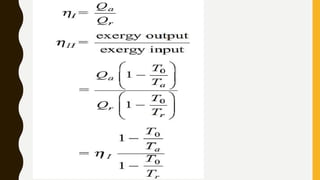1) The document discusses key concepts in thermodynamics including the limitations of the first law, heat engines, heat pumps, and parameters of performance like coefficient of performance.
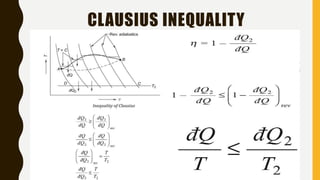
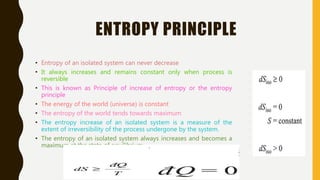
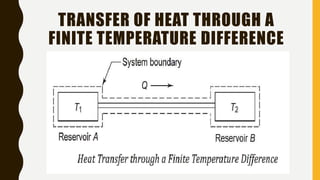
2) It also covers the second law of thermodynamics including Kelvin-Planck and Clausius statements, perpetual motion machines, and the equivalence of the statements.
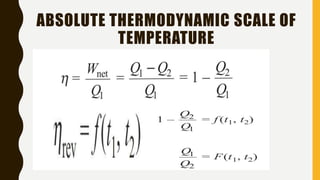
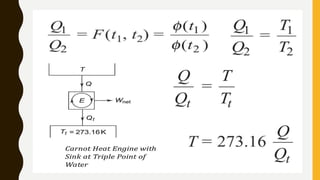
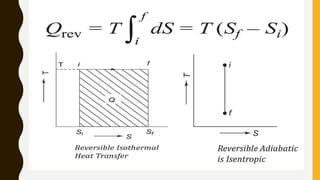
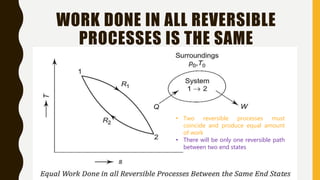
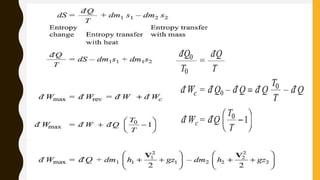
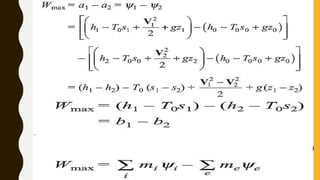
3) Additionally, it discusses Carnot's principle and cycle, the thermodynamic temperature scale, and an elementary treatment of the third law of thermodynamics.