Embed presentation
Downloaded 21 times













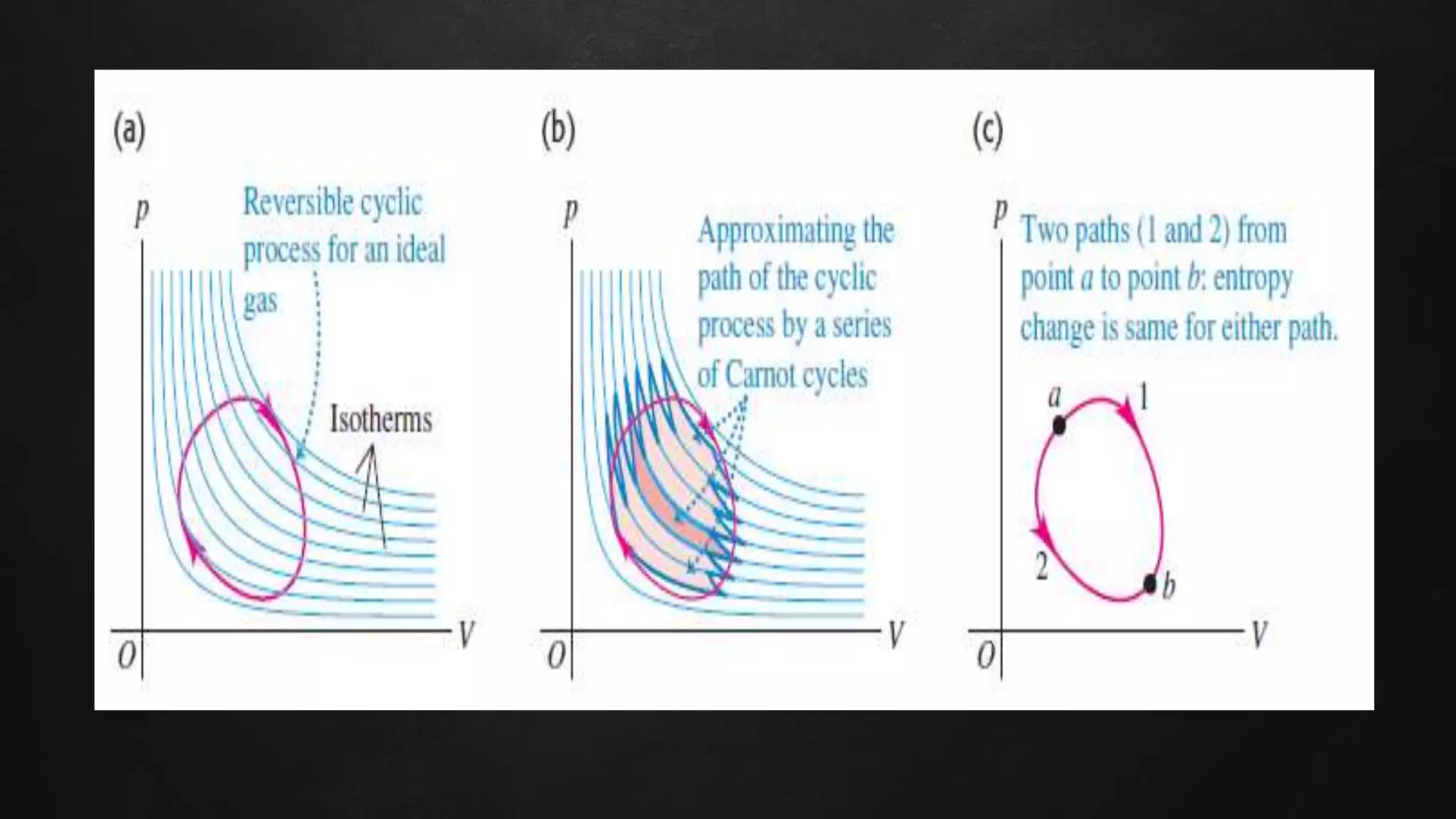
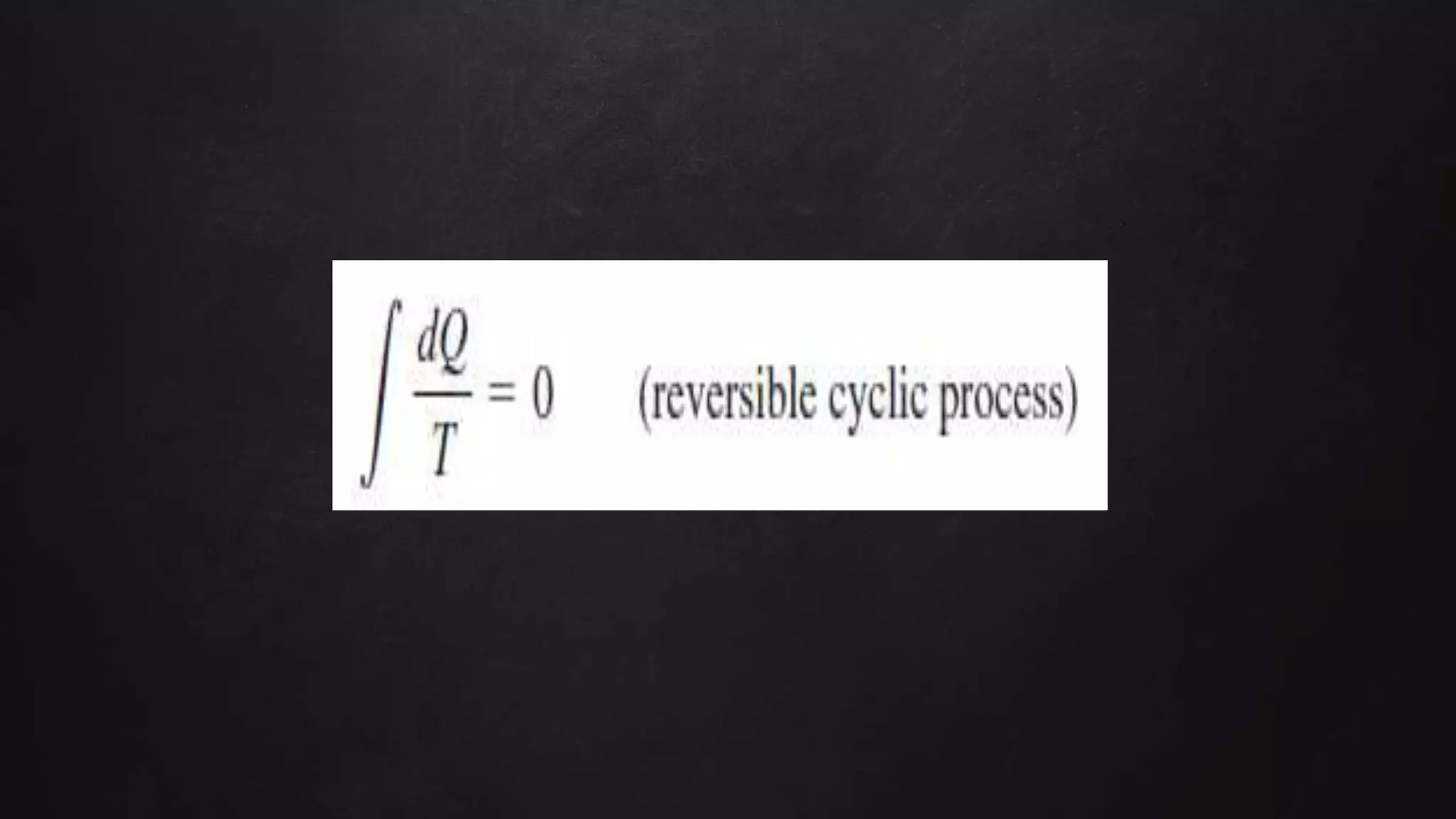
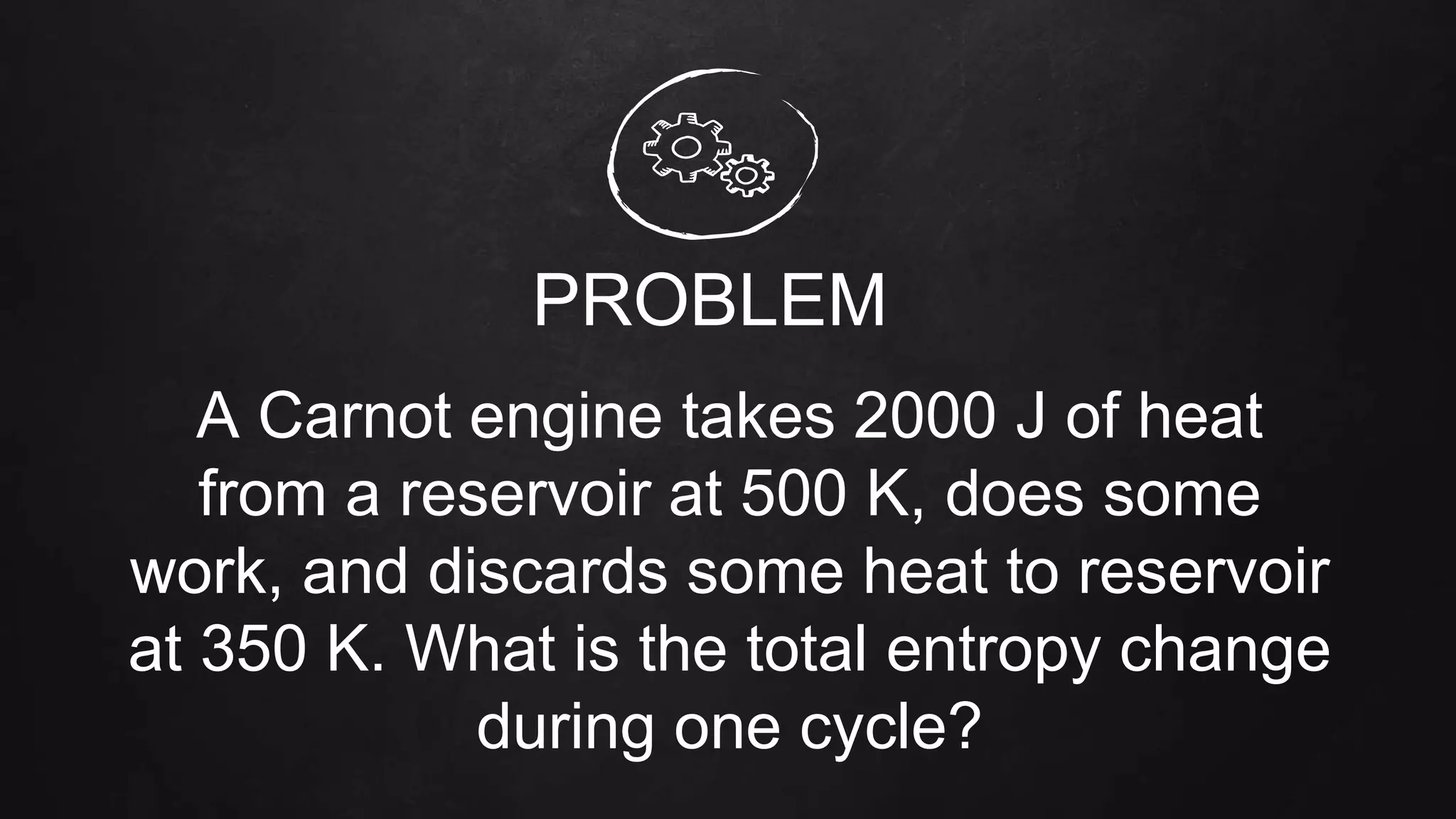




Entropy is a measure of disorder. The document discusses entropy in the context of several thermodynamic processes and calculations. These include: 1) the change in entropy of 1 kg of ice melting at 0°C, 2) the change in entropy of heating 1 kg of water from 0°C to 100°C, and 3) calculating the total entropy change for a Carnot engine that takes 2000 J of heat from a 500 K reservoir and discards heat to a 350 K reservoir.



















