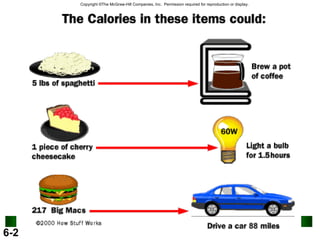
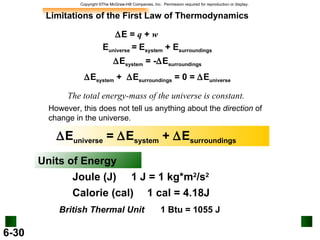
1. A liter of gasoline contains 8000 calories of energy. A person uses an average of 2000 calories per day. Excess calories are stored as fat.
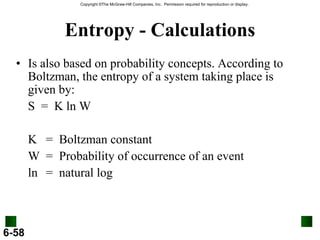
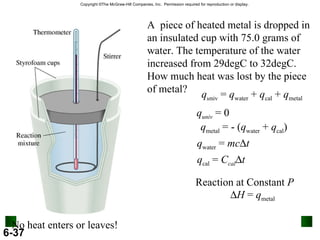
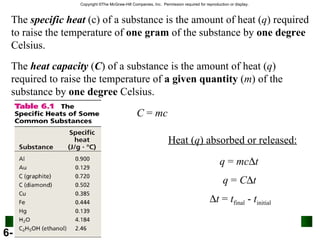
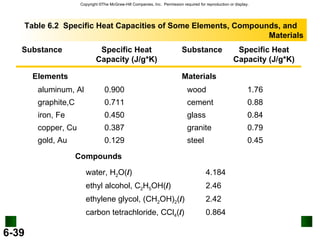
2. Calorimetry is used to determine the energy content of substances by measuring heat changes. Specific heat and heat capacity allow calculation of heat from temperature changes.
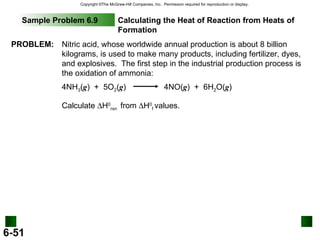
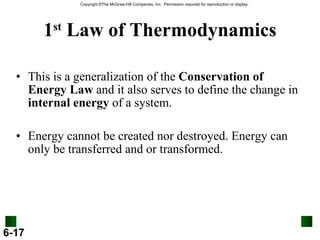
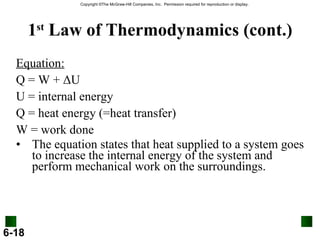
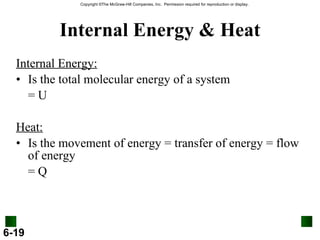
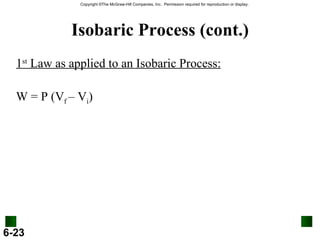
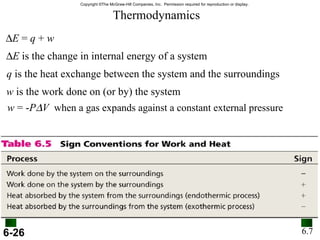
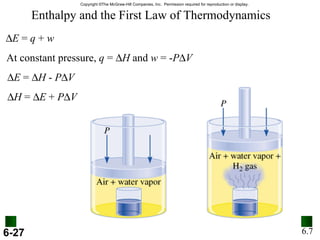
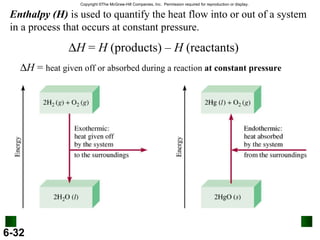
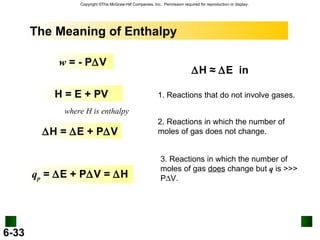
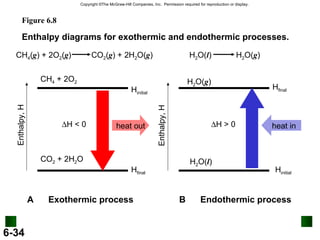
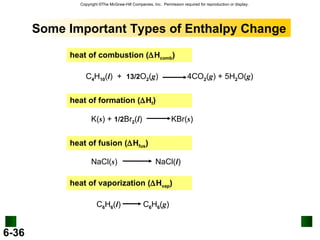
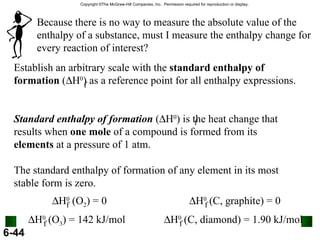
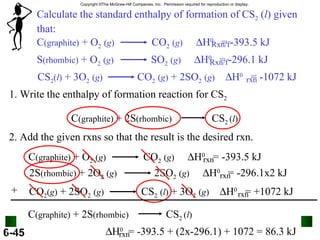
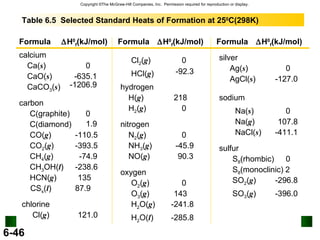
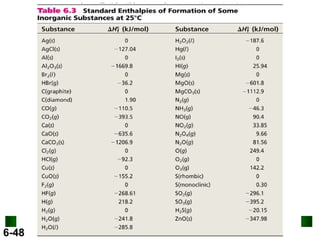
3. Enthalpy (H) quantifies heat flow during chemical reactions. Standard enthalpies of formation provide a reference scale for enthalpy values.

















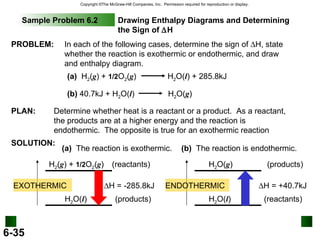




![6.5 Hess’s Law: When reactants are converted to products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps. (Enthalpy is a state function. It doesn’t matter how you get there, only where you start and end.) The standard enthalpy of reaction ( H 0 ) is the enthalpy of a reaction carried out at 1 atm. rxn a A + b B c C + d D H 0 rxn d H 0 (D) f c H 0 (C) f = [ + ] - b H 0 (B) f a H 0 (A) f [ + ] H 0 rxn n H 0 (products) f = m H 0 (reactants) f -](https://image.slidesharecdn.com/thermochem-091203055717-phpapp01/85/Thermochem-47-320.jpg)
![Benzene (C 6 H 6 ) burns in air to produce carbon dioxide and liquid water. How much heat is released per mole of benzene combusted? The standard enthalpy of formation of benzene is 49.04 kJ/mol. 6.5 2C 6 H 6 ( l ) + 15O 2 ( g ) 12CO 2 ( g ) + 6H 2 O ( l ) H 0 rxn n H 0 (products) f = m H 0 (reactants) f - H 0 rxn 6 H 0 (H 2 O) f 12 H 0 (CO 2 ) f = [ + ] - 2 H 0 (C 6 H 6 ) f [ ] H 0 rxn = [ 12x–393.5 + 6x–187.6 ] – [ 2x49.04 ] = -5946 kJ -5946 kJ 2 mol = - 2973 kJ/mol C 6 H 6](https://image.slidesharecdn.com/thermochem-091203055717-phpapp01/85/Thermochem-49-320.jpg)