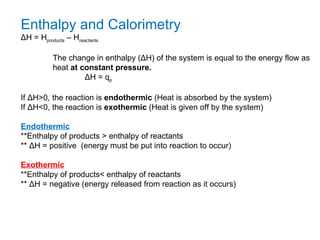
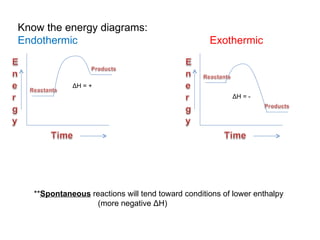
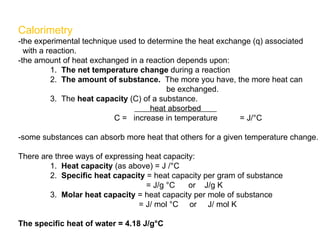
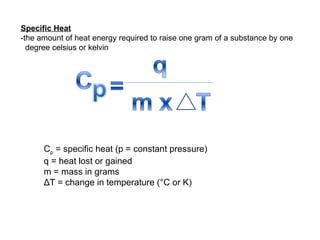
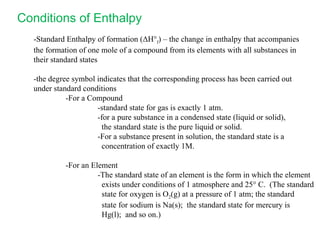
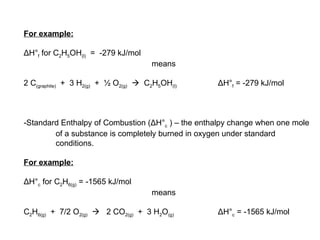
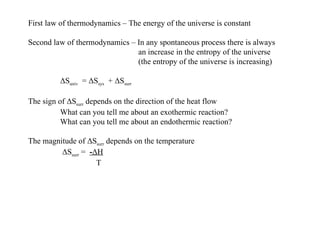
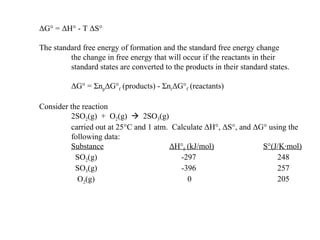
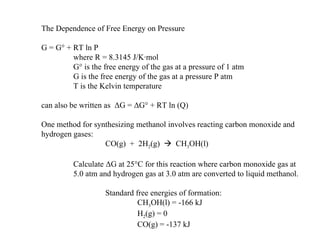
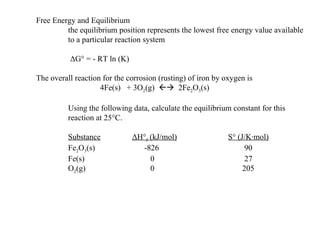
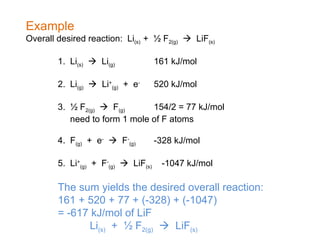
The document defines key terms in thermodynamics including system, surroundings, open system, closed system, isolated system, exothermic, endothermic, enthalpy, kinetic energy, potential energy, heat, work, state functions, standard enthalpy of formation, standard enthalpy of combustion, and entropy. It also discusses the first and second laws of thermodynamics, Gibbs free energy, and how to calculate thermodynamic properties using standard enthalpies of formation.