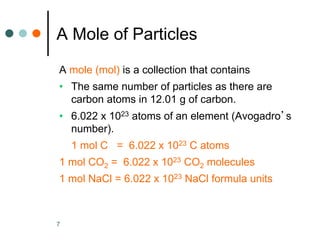
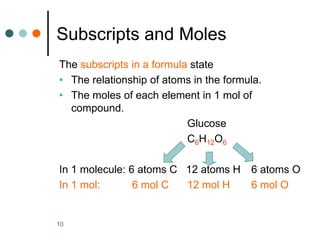
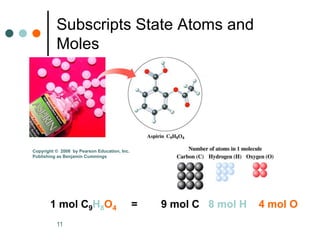
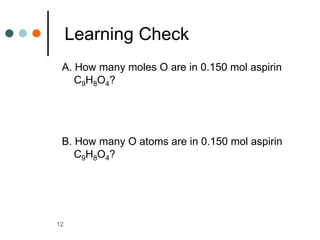
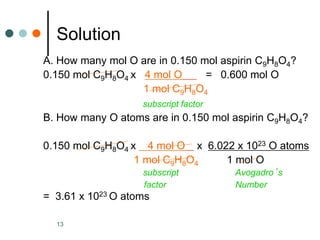
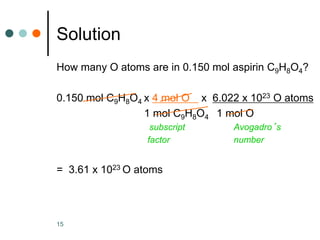
This document discusses the mole, which is a unit used to measure amounts of substances. It defines key terms related to the mole, such as Avogadro's number and molar mass. The mole allows chemists to convert between the number of particles and mass of a substance. The document explains how subscripts in chemical formulas indicate the number of atoms of each element in one molecular formula unit and in one mole of a compound. It provides an example problem calculating the number of oxygen atoms in a given number of moles of aspirin.