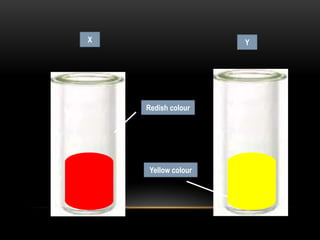
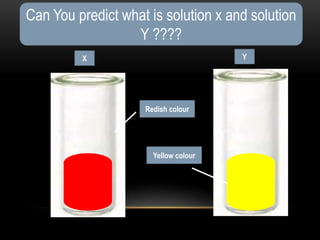
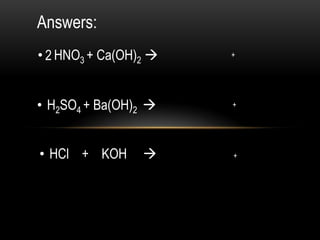
The document discusses neutralization in chemistry. It lists the group members and learning objectives which are to explain neutralization, write equations for neutralization reactions, and explain applications of neutralization. It then covers definition of neutralization, examples of neutralization equations, and applications like insect stings, indigestion, soil treatment, and factory waste neutralization.