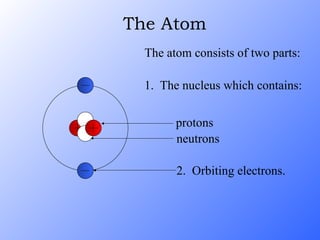
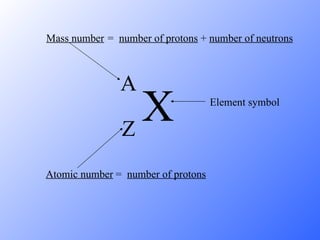
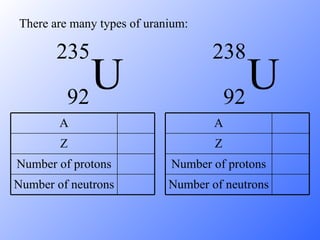
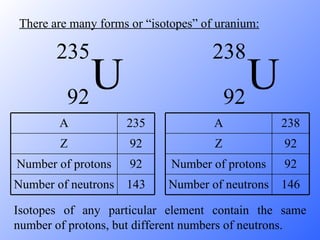
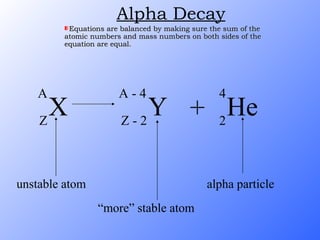
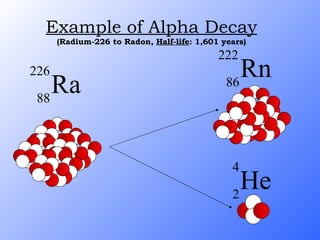
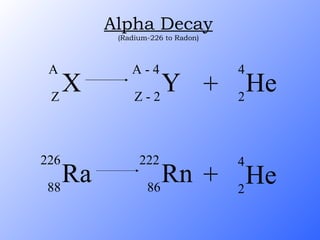
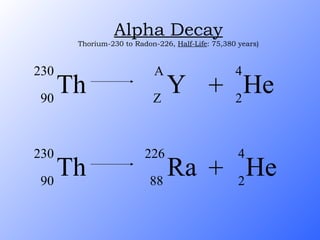
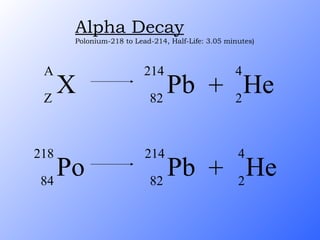
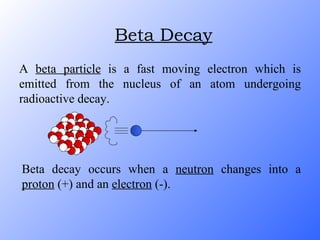
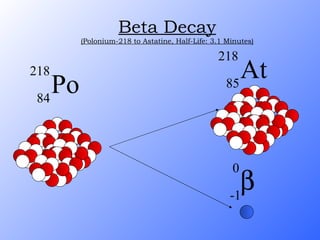
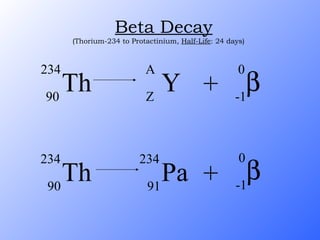
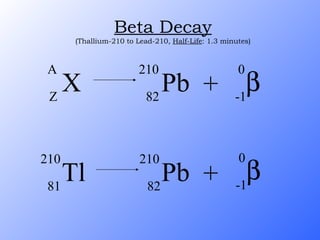
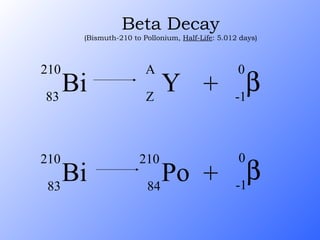
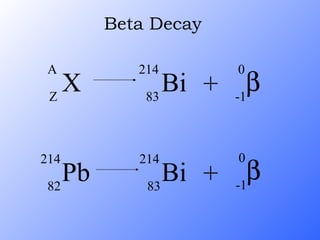
The document discusses the three main types of radioactive decay - alpha, beta, and gamma decay. It describes the particles emitted in each type of decay and how they affect the atom. It provides examples of specific radioactive isotopes, their decay processes, and half-lives. It also outlines some medical, industrial, and research applications that make use of different radioactive emitters.