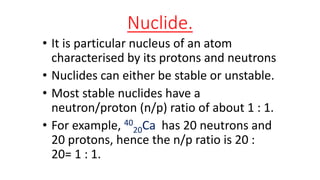
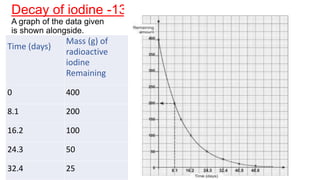
1. The document defines key terms related to radioactivity including nuclide, radioisotope, radioactive decay, and half-life.
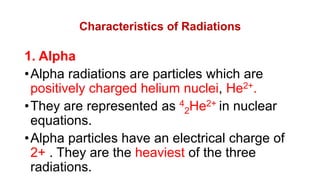
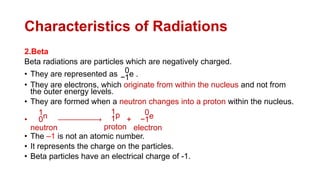
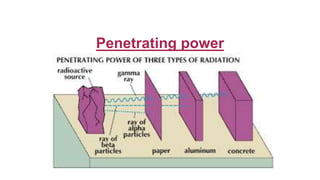
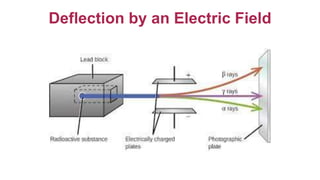
2. It describes the three main types of radiation emitted during radioactive decay - alpha particles, beta particles, and gamma rays - and their properties like penetrating power and how they are affected by electric fields.
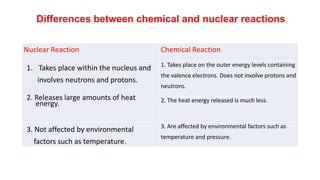
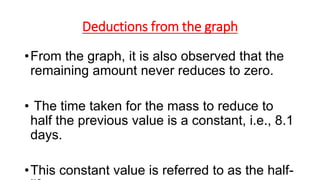
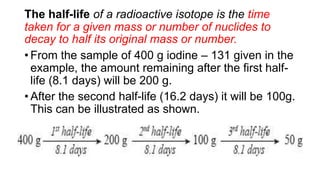
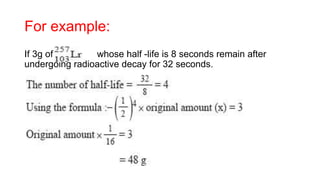
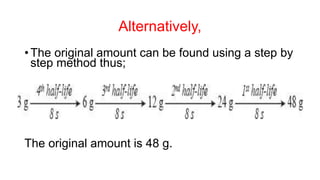
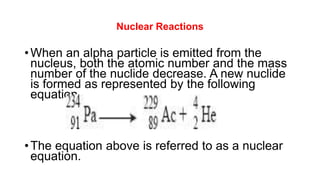
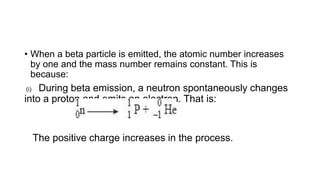
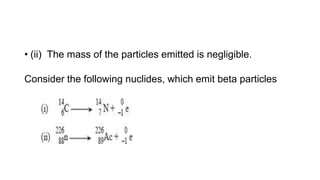
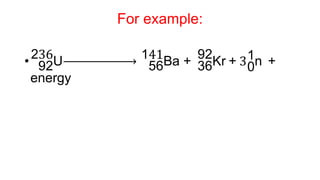
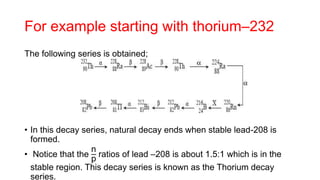
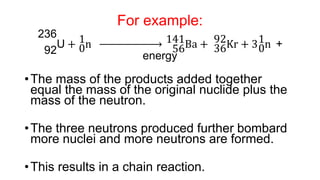
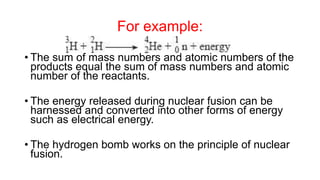
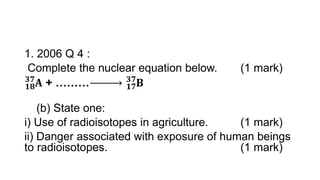
3. Equations are provided to represent nuclear reactions during radioactive decay, and examples are given of calculating radioactive decay using half-life and determining original amounts.