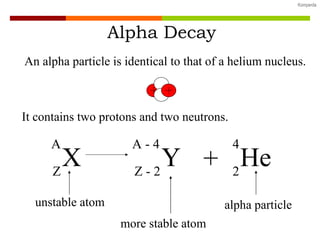
The document discusses various types of nuclear decay:
- Alpha decay involves emitting an alpha particle (helium nucleus), decreasing the atomic number by 2 and mass number by 4.
- Beta decay involves changing a neutron to a proton and emitting an electron, increasing the atomic number by 1 while keeping the same mass number.
- Electron capture involves capturing an electron by a proton to form a neutron, decreasing the atomic number by 1 while keeping the same mass number.
- Positron emission involves changing a proton to a neutron and emitting a positron, decreasing the atomic number by 1 while keeping the same mass number.
- Gamma decay involves emitting high energy gamma rays without changing mass or atomic number.