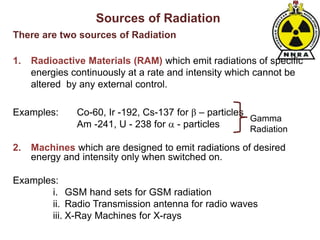
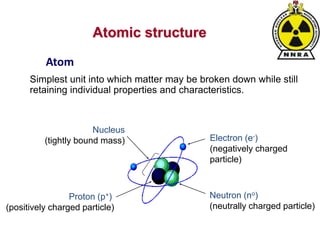
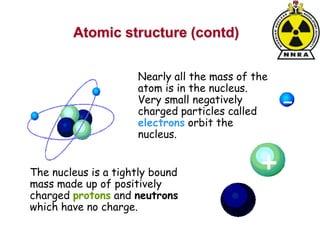
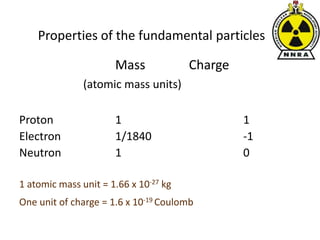
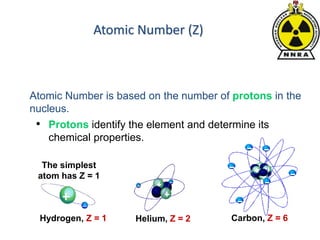
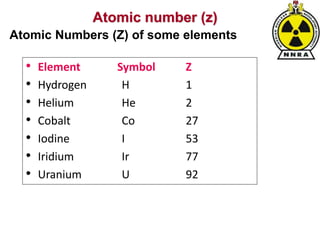
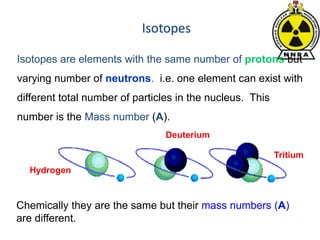
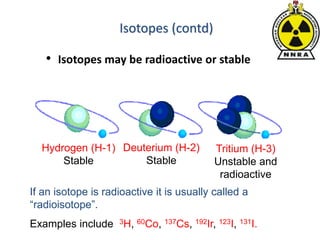
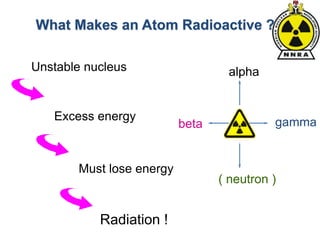
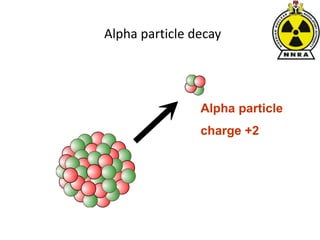
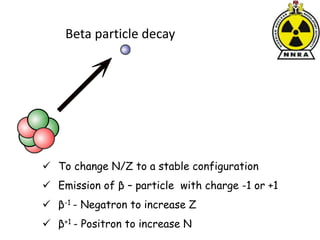
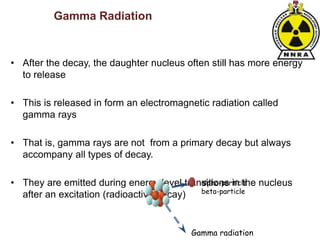
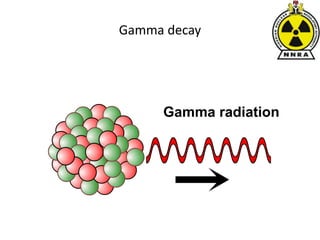
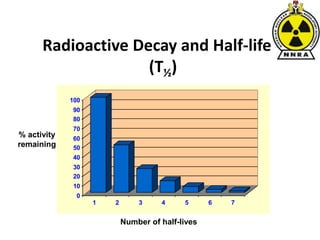
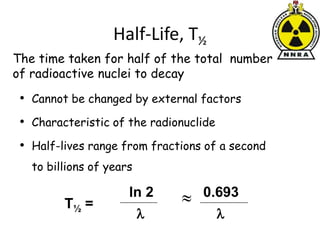
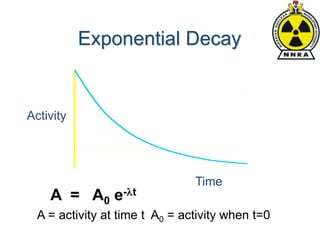
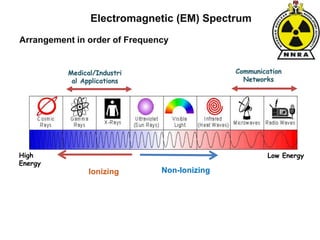
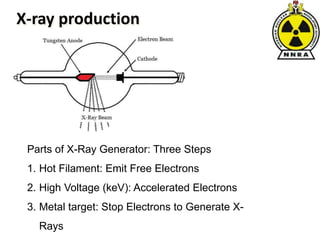
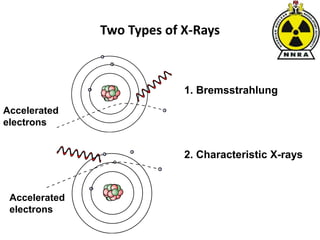
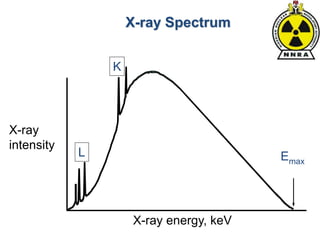
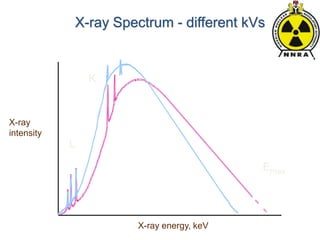
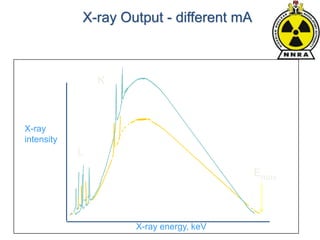
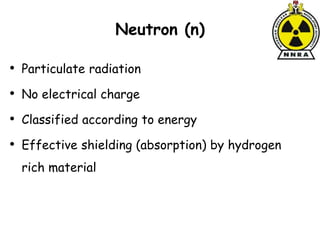
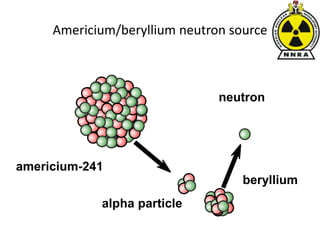
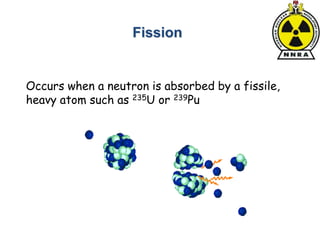
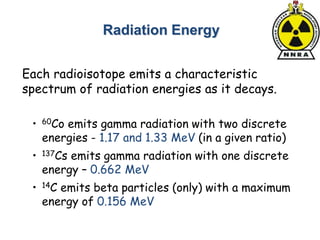
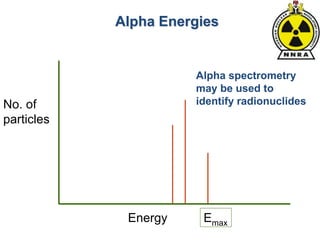
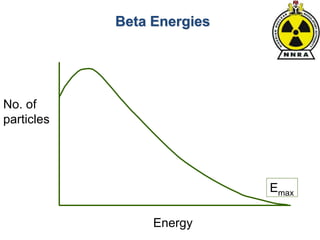
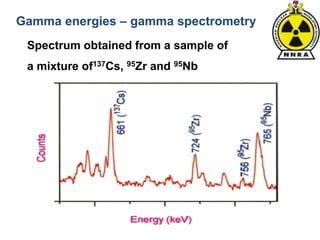
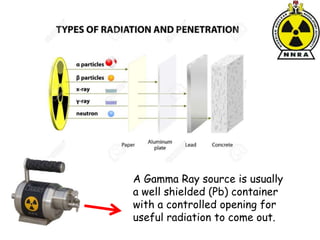
The document provides a comprehensive overview of basic nuclear physics and radioactivity, covering topics such as atomic structure, types of radiation (ionizing and non-ionizing), sources of radiation, and the mechanisms of radioactive decay. Important concepts like half-life, the behavior of isotopes, and the properties of radiation are discussed in detail, alongside practical applications in various fields. It highlights the significance of understanding radiation for radiation safety officers and its implications in daily life and industry.