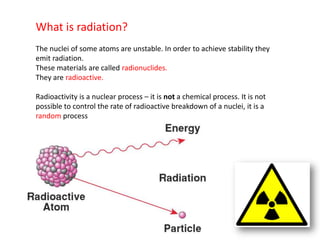
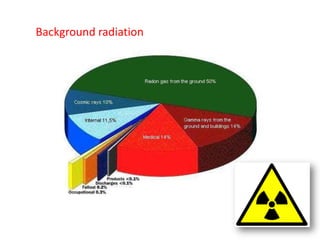
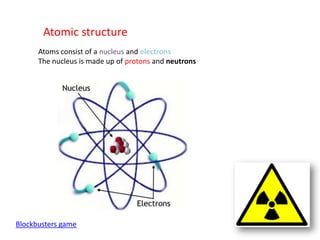
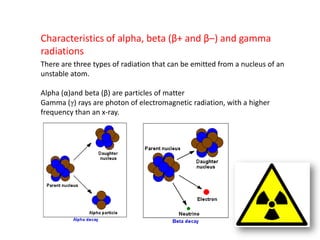
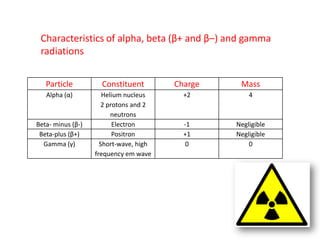
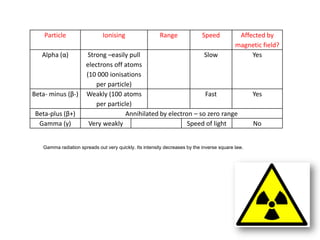
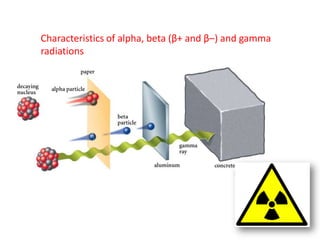
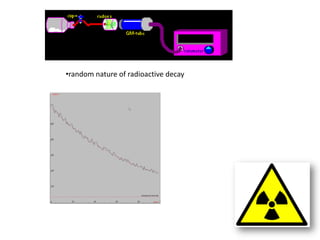
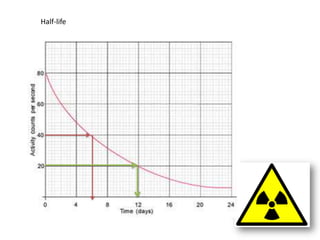
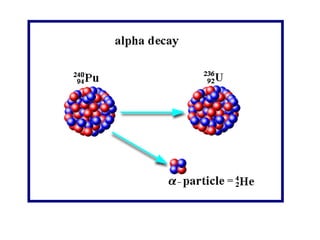
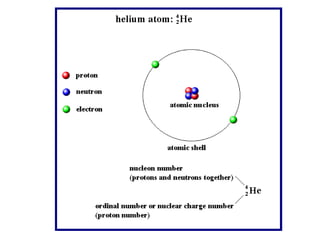
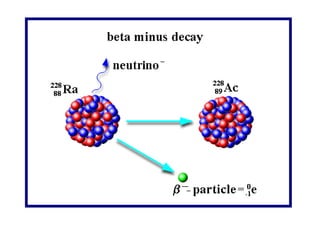
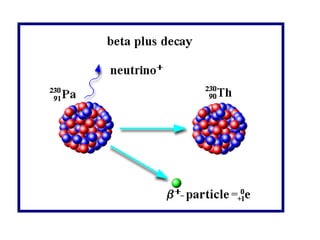
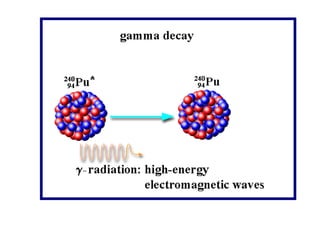
Radioactivity occurs when the nuclei of unstable atoms decay and emit radiation. There are three main types of radiation: alpha, beta, and gamma. Alpha particles consist of two protons and two neutrons, beta particles are electrons or positrons, and gamma radiation consists of electromagnetic waves. The rate of radioactive decay is random, but the half-life of a radioactive isotope is the time it takes for half the nuclei in a sample to decay. Radioactivity has industrial applications and background radiation comes from natural and man-made sources.