Embed presentation
Downloaded 153 times

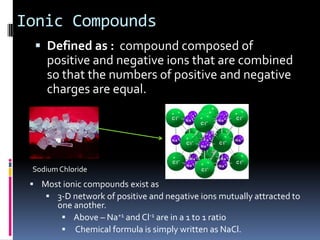
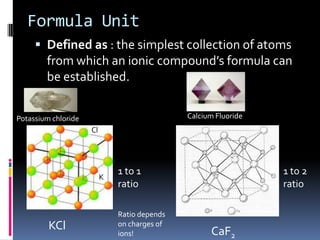
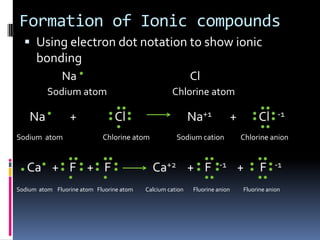
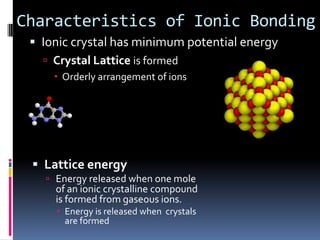
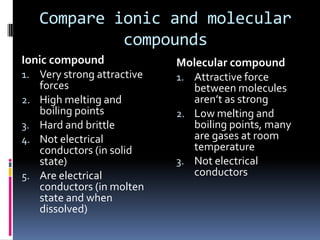
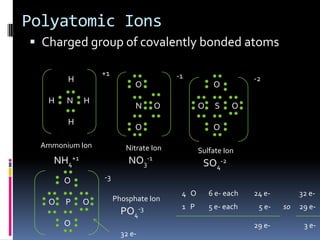
Ionic compounds are composed of positive and negative ions in ratios that balance the charges. They form crystalline lattices with ions arranged in an orderly pattern. The ions are held together by strong electrostatic forces called lattice energy. Ionic compounds have high melting and boiling points, are hard and brittle, and conduct electricity when molten or dissolved but not in the solid state. Polyatomic ions are charged groups of covalently bonded atoms that behave as single ions in ionic compounds.







