Embed presentation
Downloaded 266 times


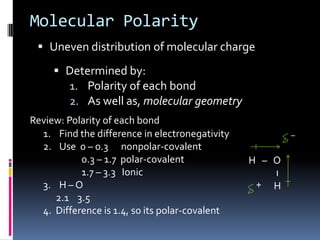

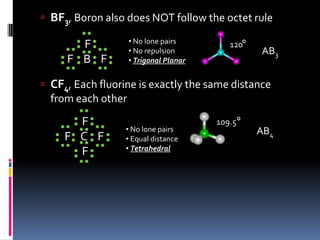
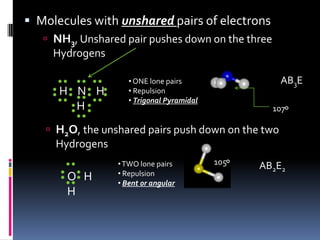
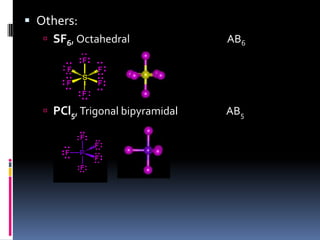

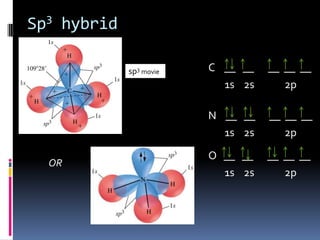
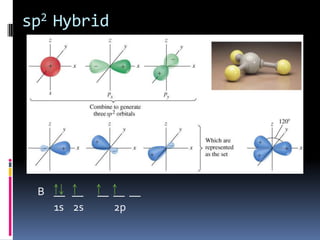
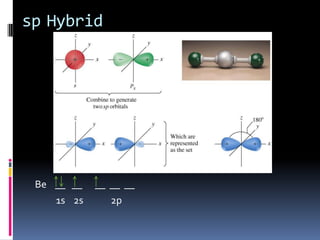
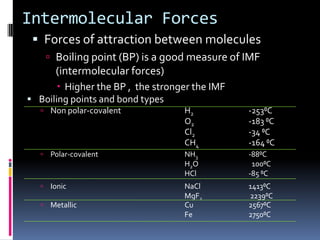
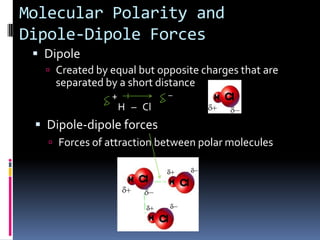
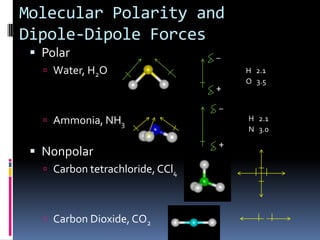
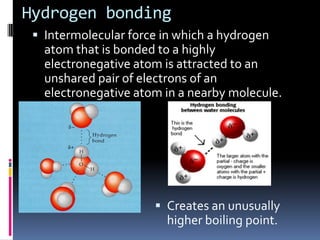
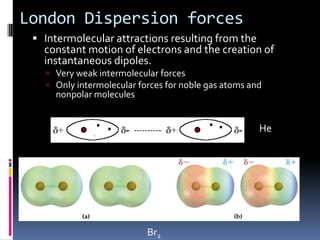
This document discusses molecular geometry and polarity using valence shell electron pair repulsion (VSEPR) theory and hybridization theory. It begins by explaining the objectives and defining molecular polarity in terms of bond polarity and molecular geometry. It then applies VSEPR theory to predict the shapes of different molecules and polyatomic ions. The document continues by introducing hybridization theory to explain bonding orbitals and different hybridizations like sp3, sp2, and sp. It concludes by describing various intermolecular forces like dipole-dipole forces, hydrogen bonding, London dispersion forces, and how they relate to boiling points.















