1. Ionic compounds form when oppositely charged ions attract each other to form electrically neutral compounds. The attraction between oppositely charged ions is called an ionic bond.
2. Many ionic compounds are binary, containing only two different elements. Sodium chloride is an example as it contains sodium and chlorine.
3. Ionic bonds produce crystalline structures where each positive ion is surrounded by negative ions and vice versa. These strong bonds give ionic compounds high melting and boiling points.

![Compound formation and charge
Example: (calcium fluoride )
6- Calcium has the electron configuration [Ar] 4s 2 , and needs to
lose two electrons to attain the stable configuration of argon.
Fluorine has the configuration [He]2 s 2 2p 5 , and must gain one
electron to attain the stable configuration of neon. Because the
number of electrons lost and gained must be equal, two fluorine
atoms are needed to accept the two electrons lost from the
calcium atom. he overall charge of one unit of calcium fluoride
(CaF2 ) is zero
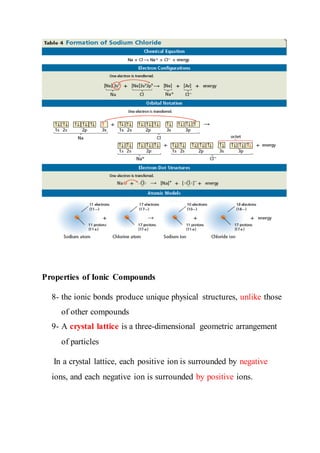
7- formation of an ionic compound such as sodium chloride can
be represented](https://image.slidesharecdn.com/answeredionicbondsandioniccompounds-170316095228/85/Answered-ionic-bonds-and-ionic-compounds-2-320.jpg)