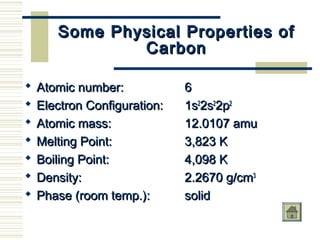
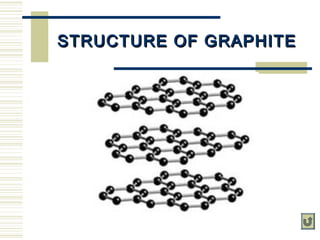
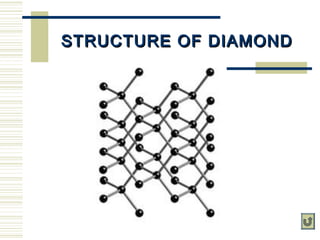
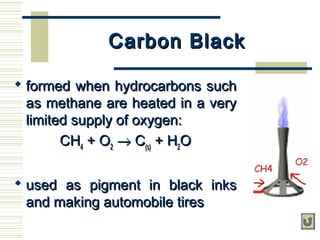
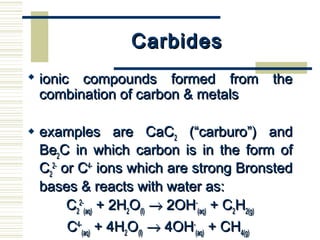
Carbon exists in several allotropes with unique properties. Graphite has layered structures that allow for easy sliding of layers and is used as lubricant and pencil lead. Diamond has a tetrahedral structure and is the hardest material. Fullerenes like buckminsterfullerene have soccer ball shapes. Carbon also forms many inorganic compounds including carbon monoxide, carbon dioxide, carbonates, bicarbonates, carbides and cyanides that have various applications. Organic chemistry is the study of carbon compounds.