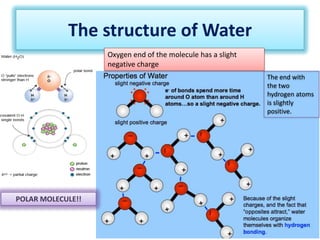
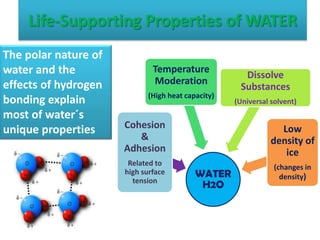
Water has unique properties that support life due to its molecular structure and ability to form hydrogen bonds. The polar nature of the water molecule, with slightly positive and negative ends, allows it to form hydrogen bonds with other water molecules. This gives water high surface tension, adhesion, cohesion, heat capacity, and the ability to dissolve many substances. It also explains why ice floats on liquid water, maintaining the viability of aquatic ecosystems.