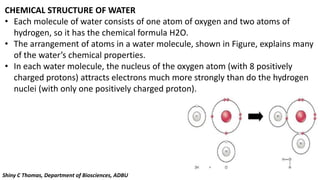
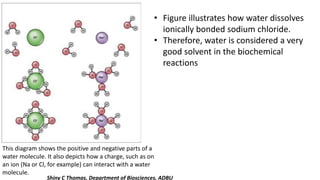
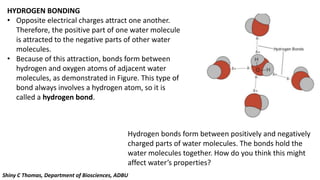
Water is essential for all known forms of life and exhibits unique properties due to its molecular structure and polarity. These properties include being a good solvent, having a high boiling point, high specific heat, cohesion, adhesion, and unusual density characteristics that enable ice to float on liquid water. These traits play crucial roles in biological processes and the survival of organisms on Earth.