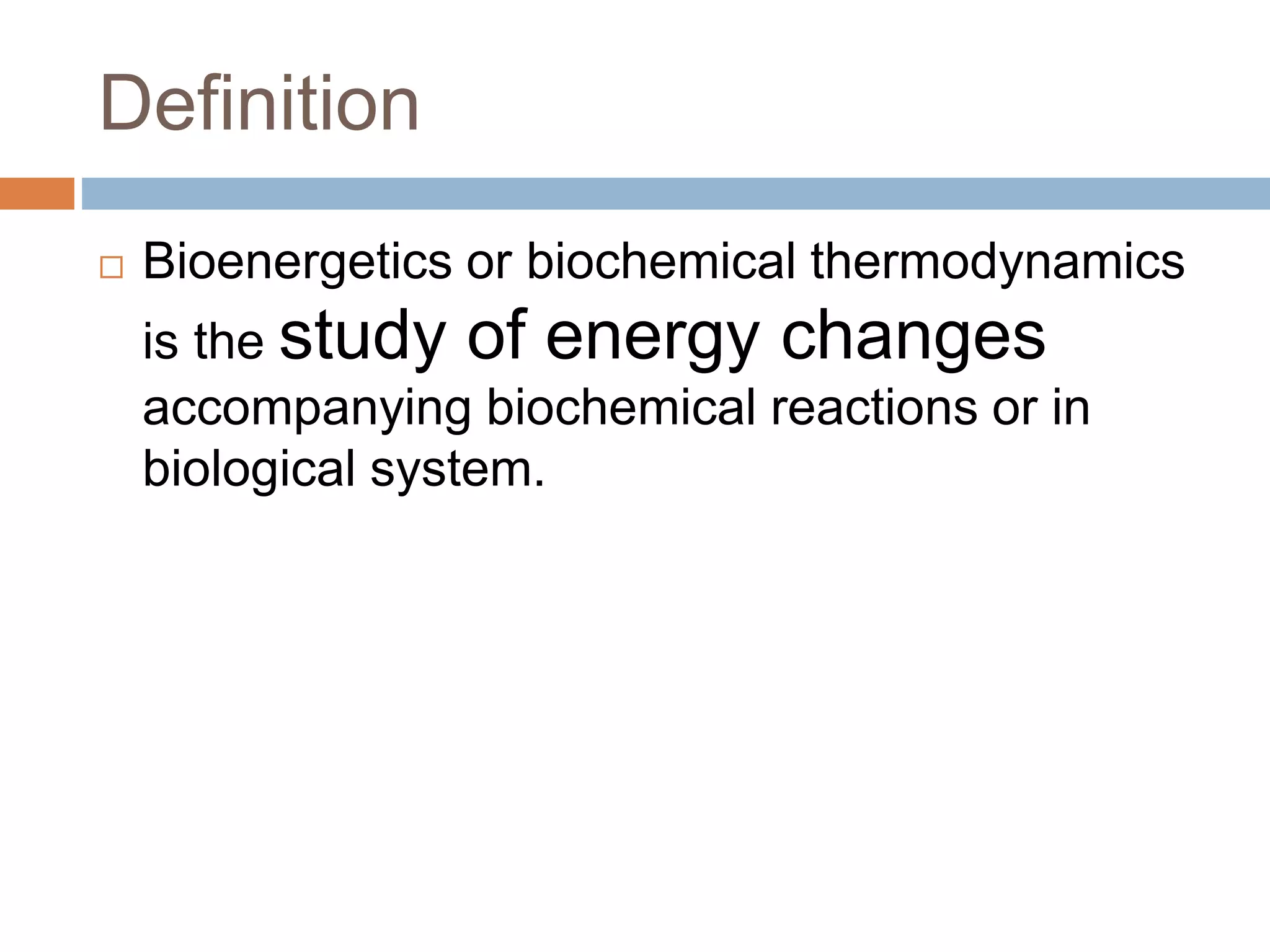
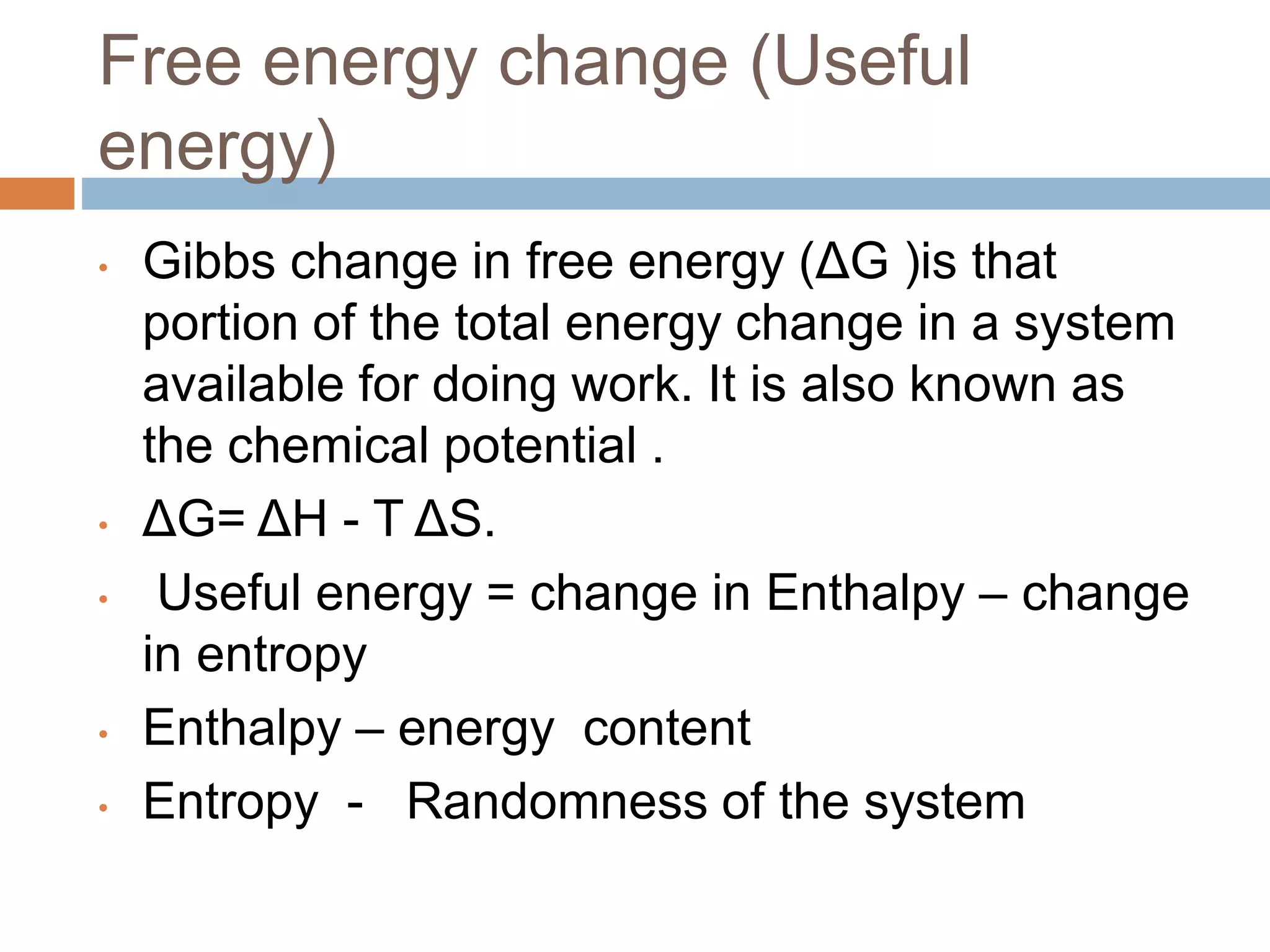
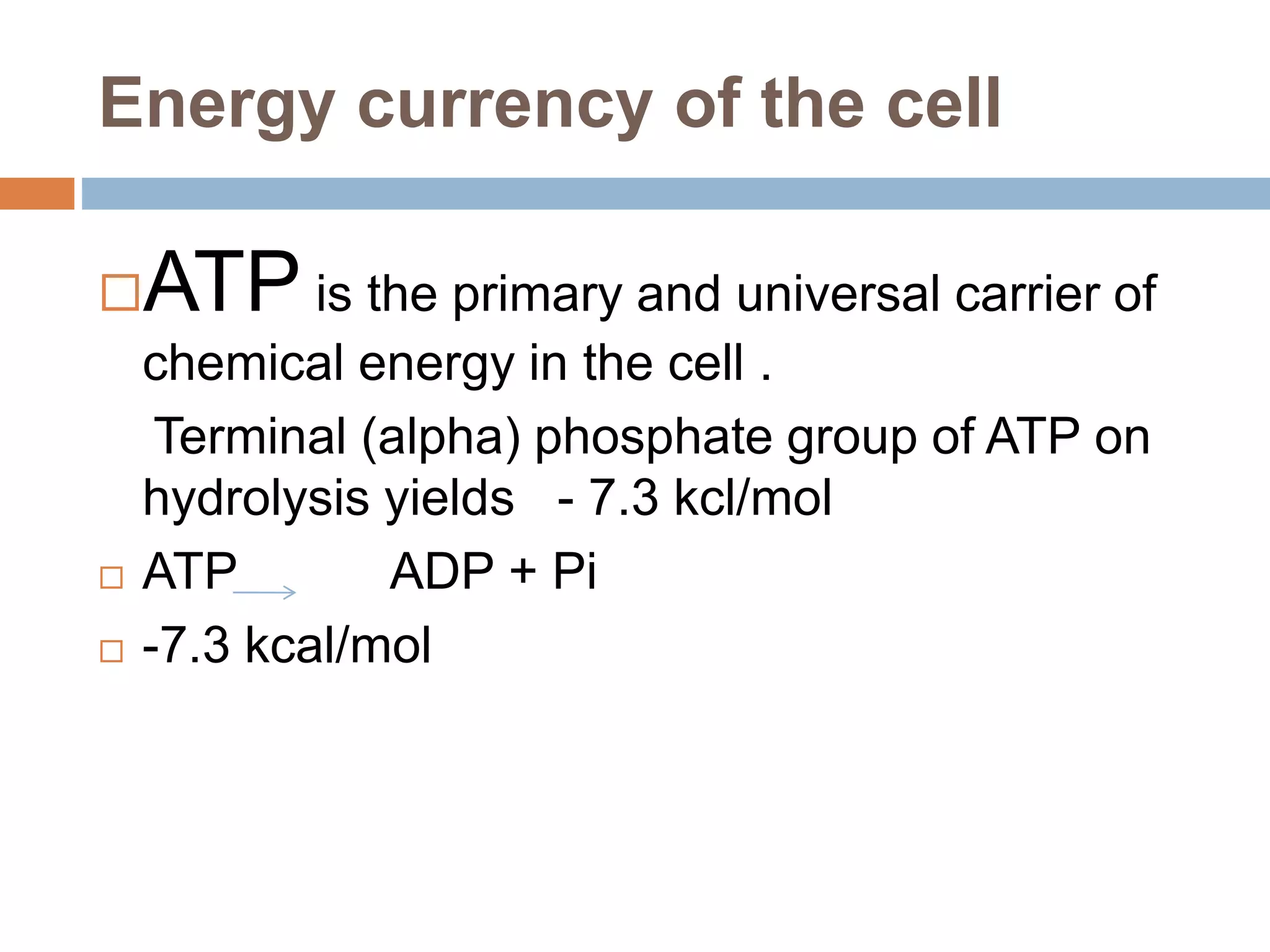
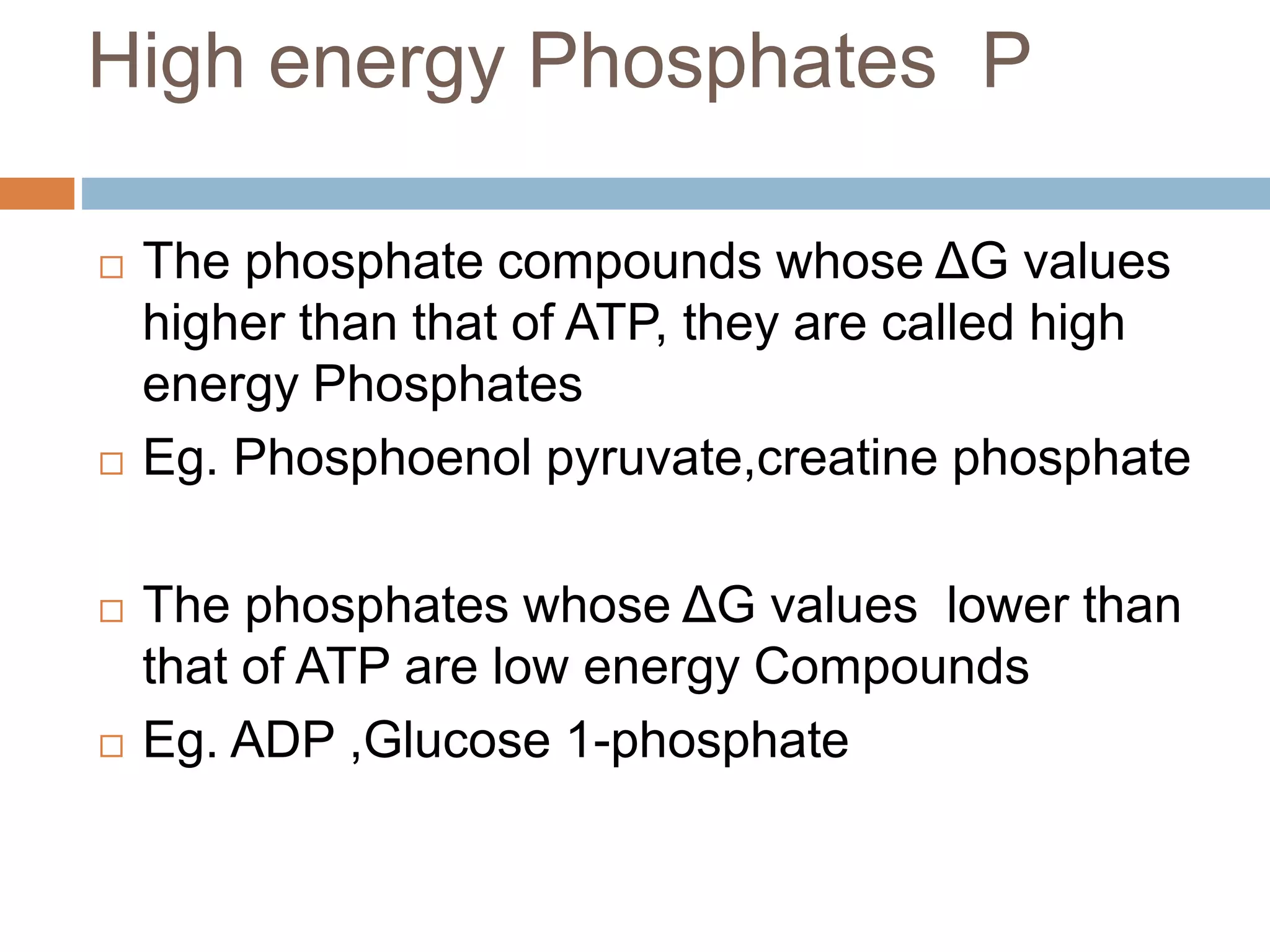
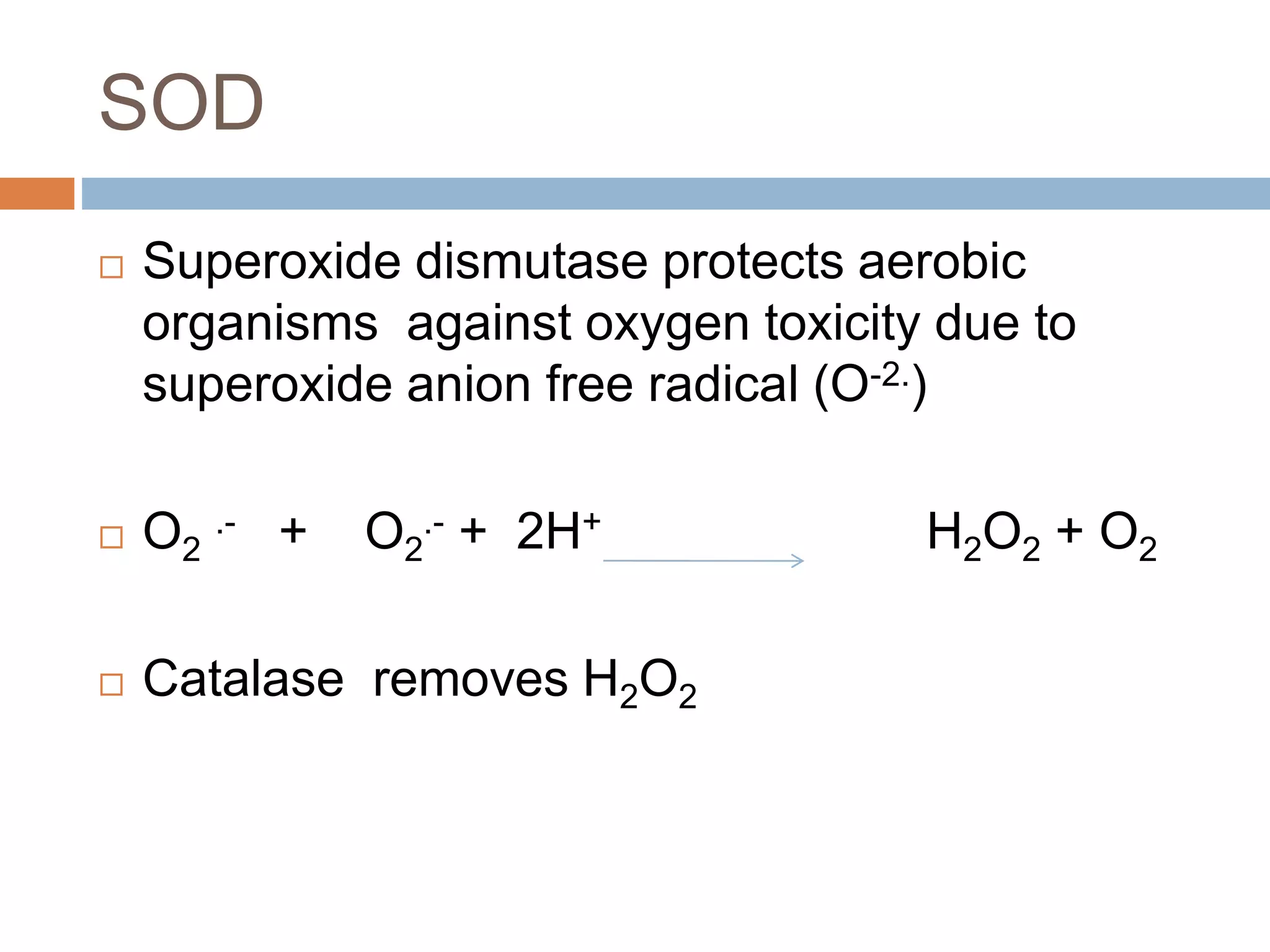
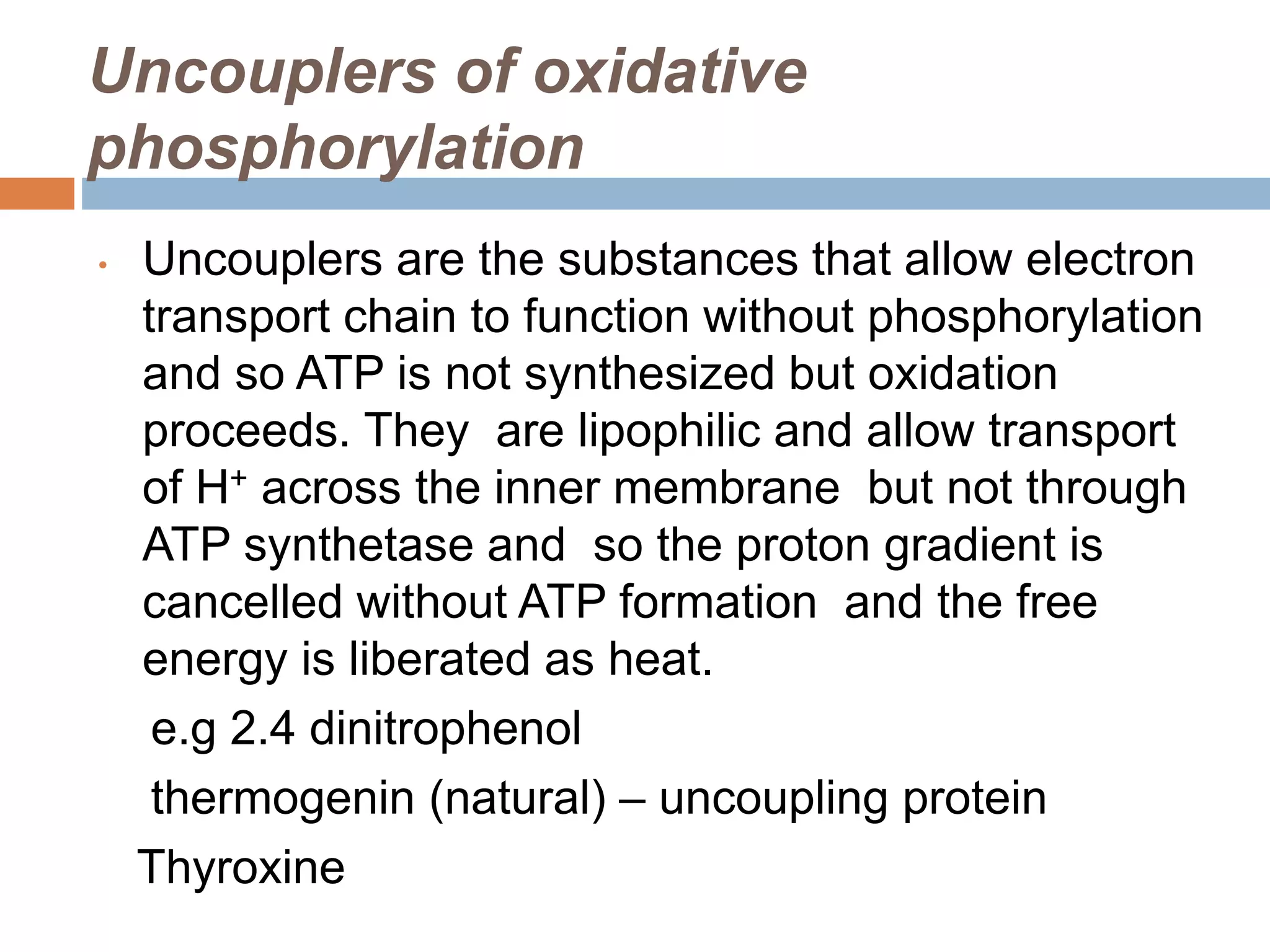
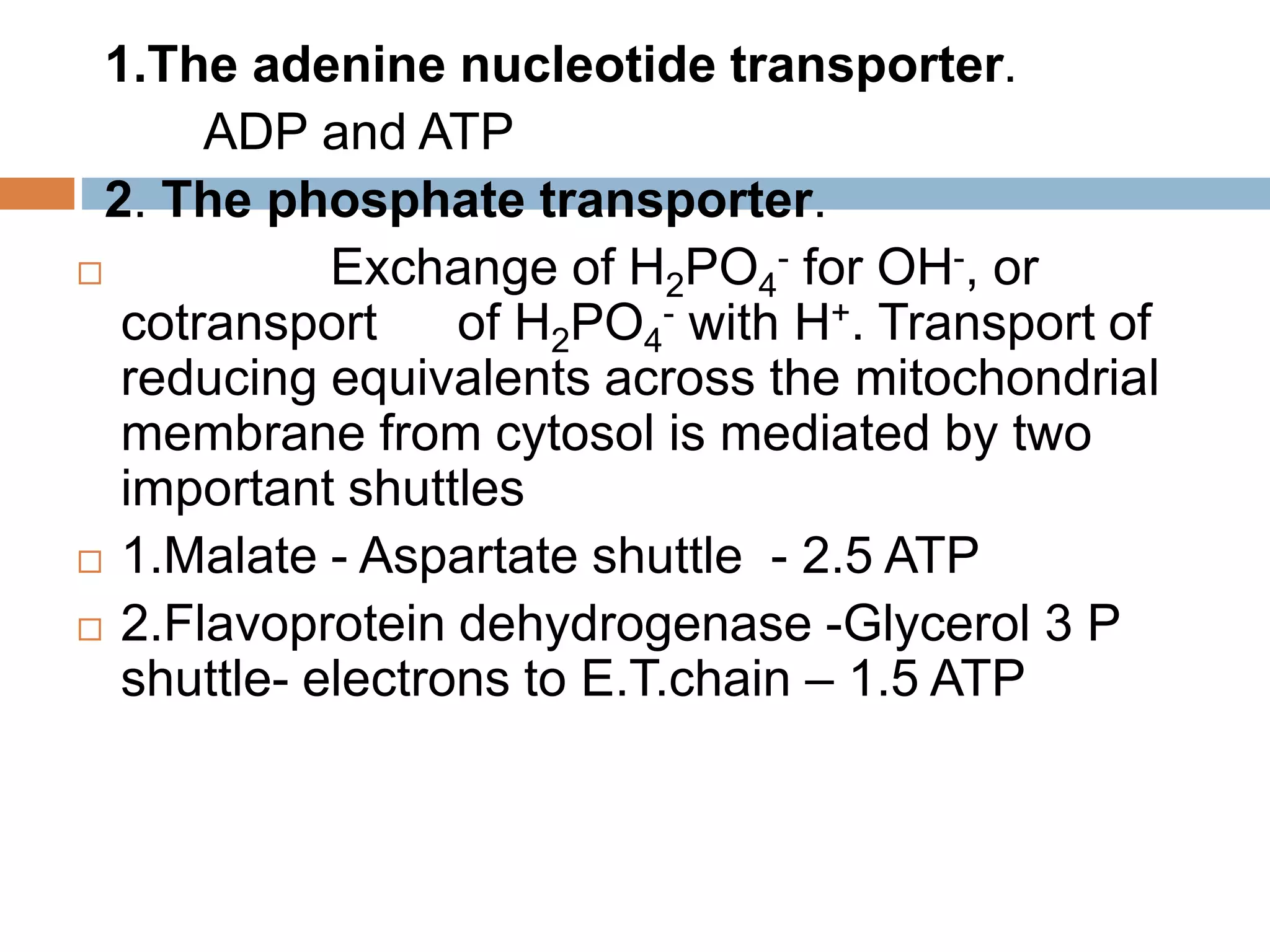
Bioenergetics is the study of energy changes in biochemical reactions and biological systems. The laws of thermodynamics govern energy changes. ATP is the primary energy currency in cells. It is produced through oxidative phosphorylation where the energy released from redox reactions is used to pump protons across the mitochondrial inner membrane, creating a proton gradient. ATP synthase uses this proton gradient to phosphorylate ADP, producing ATP. Diseases can result from defects in the electron transport chain or oxidative phosphorylation.