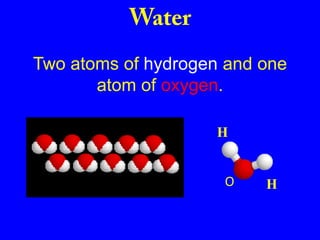
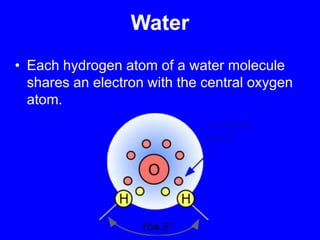
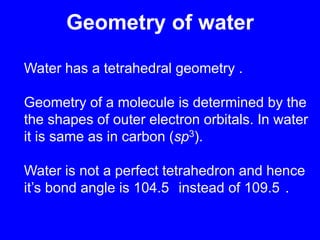
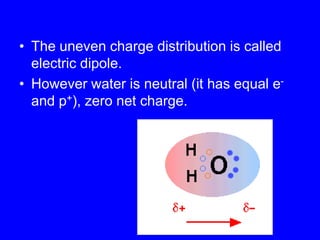
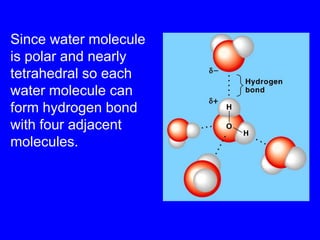
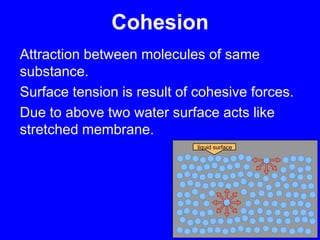
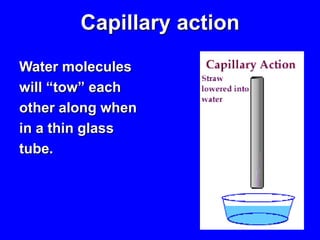
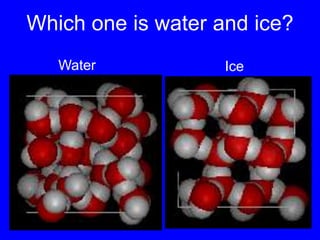
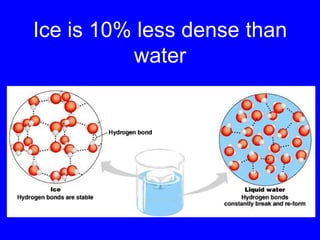
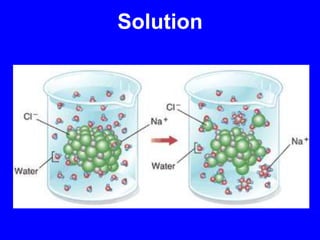
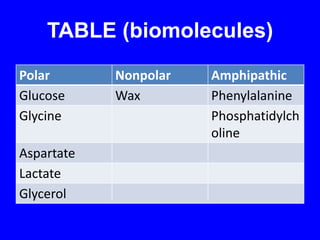
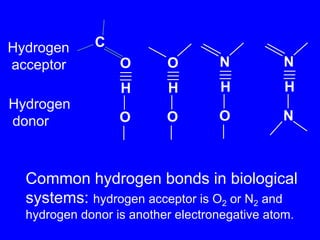
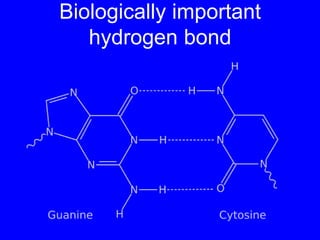
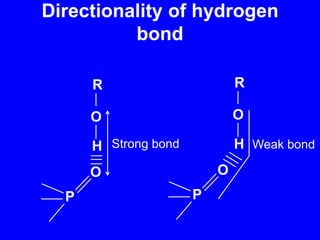
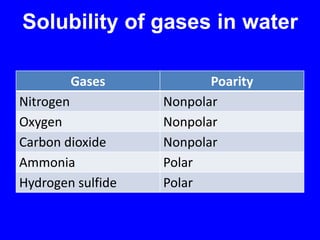
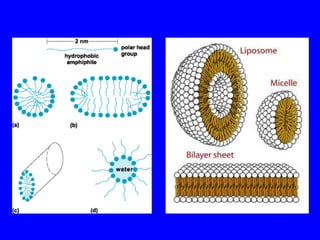
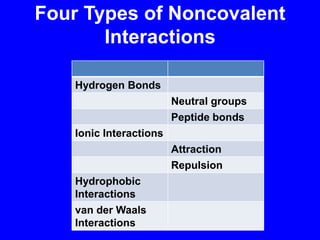
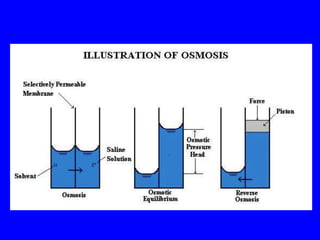
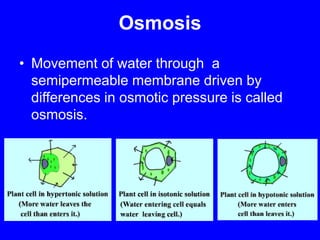
This document provides an overview of biochemistry by discussing the properties of water and its importance for life. It notes that water is a polar molecule that can form hydrogen bonds, giving it unique properties like high heat capacity and surface tension. These hydrogen bonds allow water to dissolve many polar substances. Water's hydrogen bonding and ability to dissolve biomolecules make it essential as the universal solvent for living organisms. The document also briefly discusses solutions, solutes, solvents, and how water interacts with charged and nonpolar compounds.