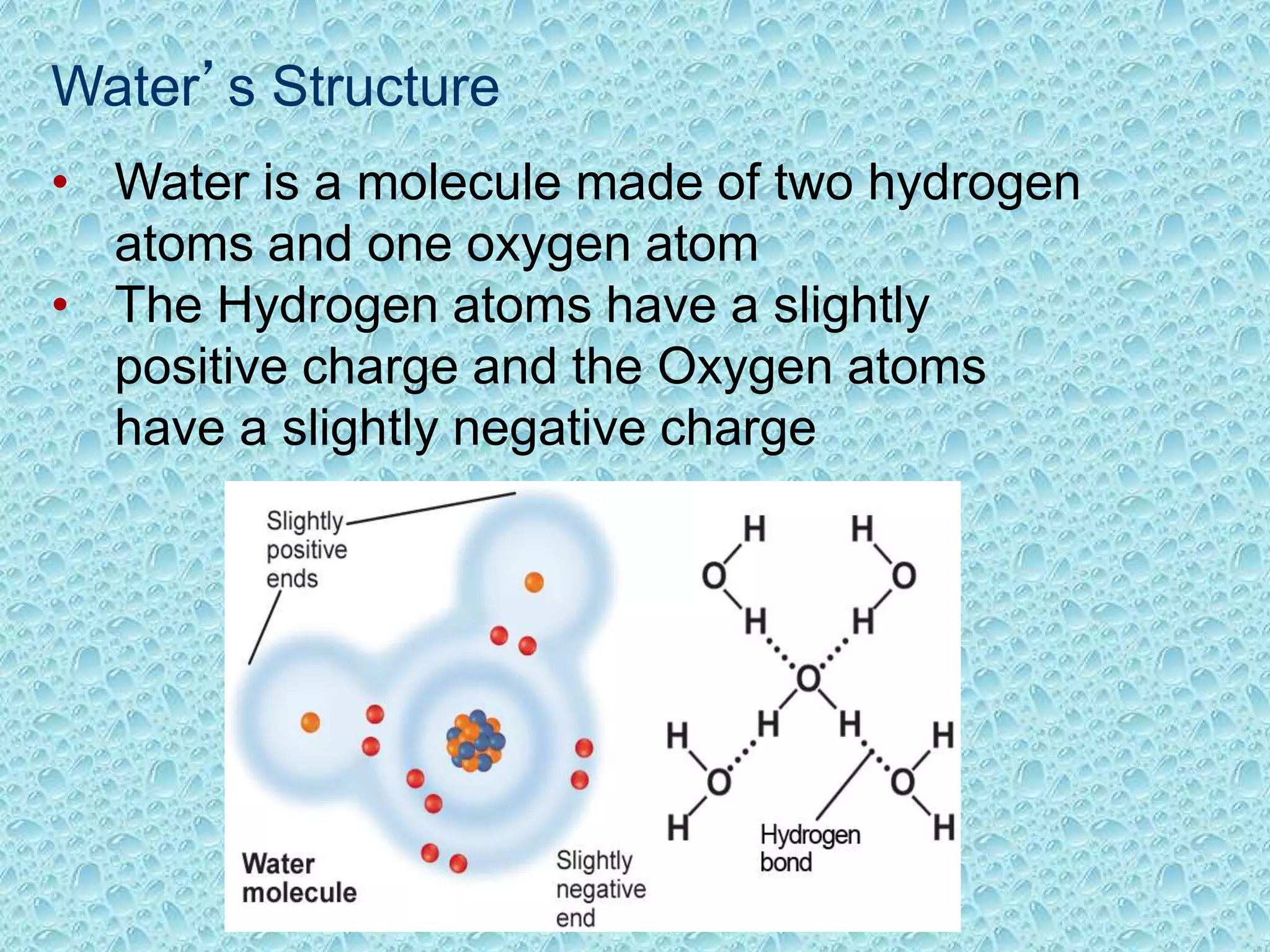
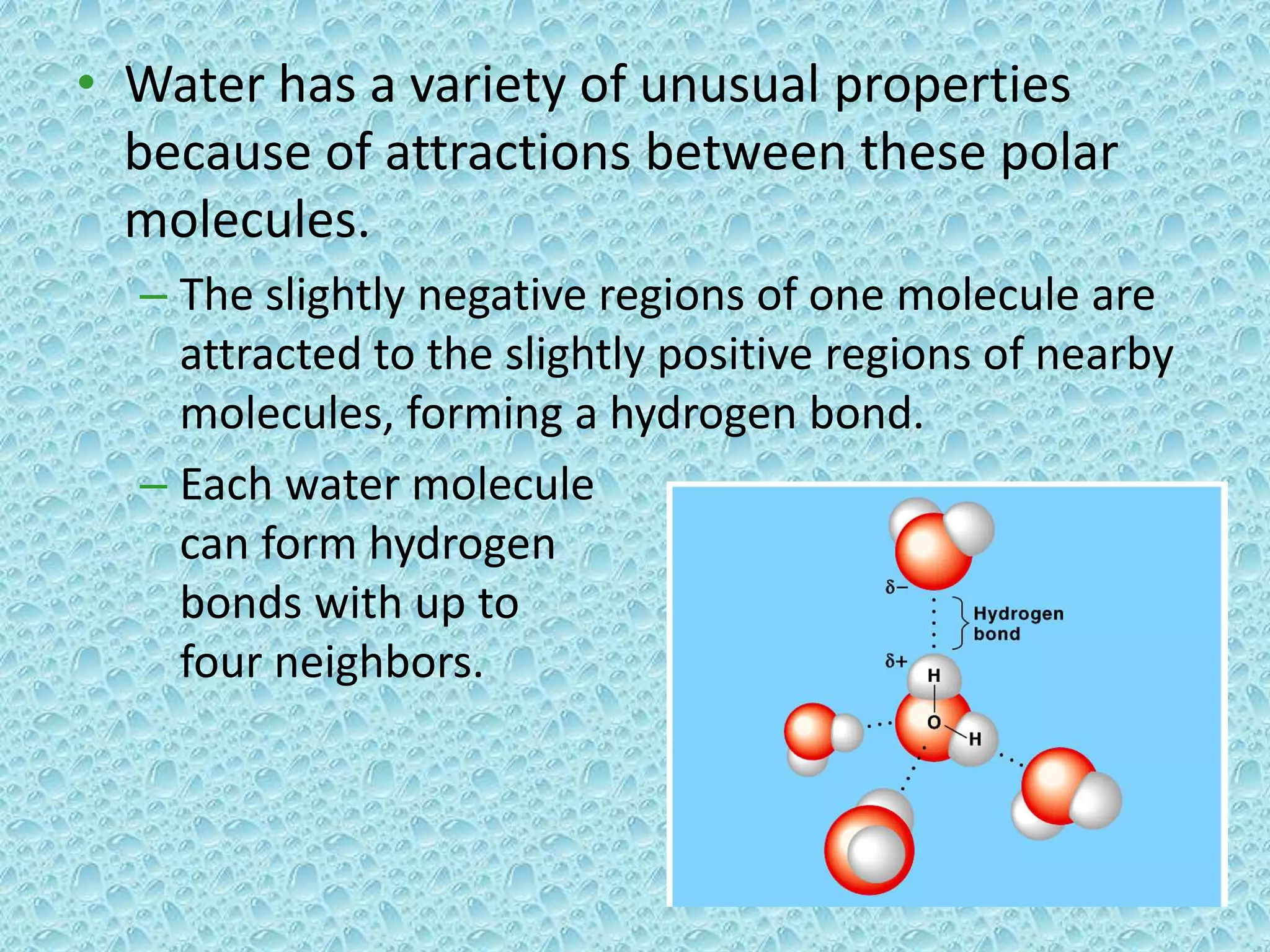
Water is made of two hydrogen atoms and one oxygen atom in a polar covalent bond. This polarity allows water molecules to form hydrogen bonds with up to four neighboring molecules. These hydrogen bonds give water its unique properties including high surface tension, heat capacity, and ability to moderate temperatures. Water's properties like cohesion, density maximum at 4°C, and solvent abilities support life by transporting nutrients in plants, stabilizing ecosystems, and allowing biochemical reactions to occur.