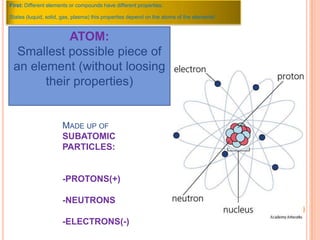
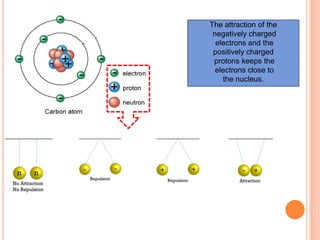
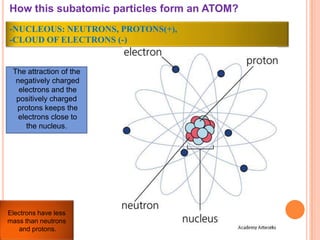
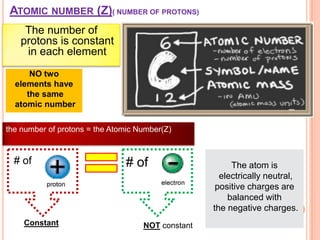
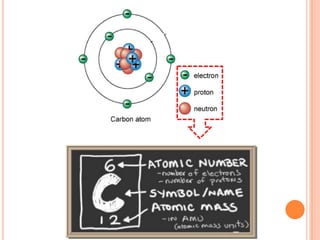
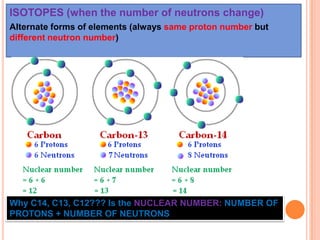
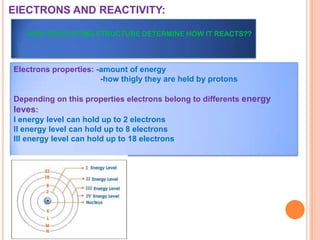
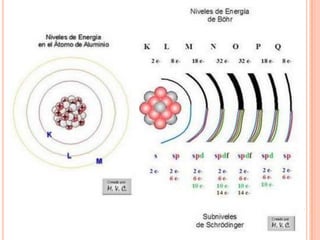
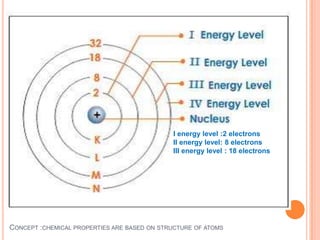
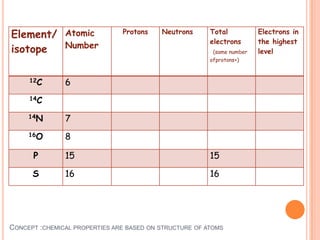
This document discusses the structure of atoms and their subatomic particles. It explains that atoms are made up of protons, neutrons, and electrons, with the nucleus containing protons and neutrons at the center and electrons orbiting around it. The document also discusses isotopes, which are variants of the same element that differ in their number of neutrons. It notes that the number of protons determines an element's atomic number and properties. Finally, it explains that an atom's structure determines how it reacts chemically based on how tightly its electrons are held.