IB Chemistry on Infrared Spectroscopy
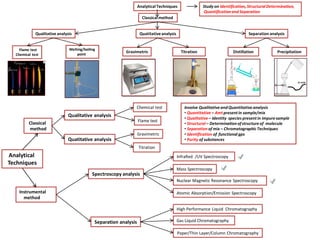
- 1. Classicalmethod Analytical Techniques Classical method Qualitative analysis Quatitative analysis Chemical test Flame test Titration Gravimetric Instrumental method Spectroscopy analysis Separation analysis Nuclear Magnetic Resonance Spectroscopy Atomic Absorption/Emission Spectroscopy InfraRed /UV Spectroscopy Mass Spectroscopy High Performance Liquid Chromatography Gas Liquid Chromatography Paper/Thin Layer/Column Chromatography AnalyticalTechniques QuatitativeanalysisQualitative analysis Separation analysis Flame test Chemical test Melting/boiling point Gravimetric Titration Distillation Precipitation Study on Identification,StructuralDetermination, Quantification and Separation Involve Qualitative and Quantitative analysis • Quantitative – Amt present in sample/mix • Qualitative – Identity species present in impure sample • Structural – Determination ofstructure of molecule • Separation of mix – Chromatographic Techniques • Identification of functional gps • Purity of substances
- 2. • Spectroscopymeasuresinteractionof moleculeswith electromagneticradiation • Particles(molecule,ion, atom) can interact/absorba quantumof light Spectroscopy Electromagnetic Radiation Nuclear spin High Energy Radiation Gamma/X ray Transitionof innerelectrons UV or visible Transitionof outermost valenceelectrons Infrared Molecularvibration Microwave Molecularrotation Radiowaves Low Energy Radiation InfraredSpectroscopy Nuclear MagneticResonance Spectroscopy Ultra Violet Spectroscopy Atomic Absorption Spectroscopy Velocity of light (c ) = frequency (f) x wavelength (λ) - c = f λ • All electromagnetic waves travel at speed of light (3.00 x 108 ms-1 ) • Radiation with high ↑ frequency – short ↓ wavelength • Electromagnetic radiation/photon carry a quantum of energy given by E = hf hc E h = plank constant = 6.626 x 10-34 Js f = frequency λ = wavelength Click here notes spectroscopy
- 3. ElectromagneticRadiationand Spectroscopy Radiowaves Nuclear spin Nuclear MagneticResonance Spectroscopy • Organic structure determination • MRI and body scanning Infrared Molecularvibration InfraredSpectroscopy UV or visible Transitionof outervalenceelectron • Organic structure determination • Functional gp determination • Measure bond strength • Measure degree unsaturationin fat • Measure level of alcohol in breath ElectromagneticRadiation UV Spectroscopy Atomic A Spectroscopy • Quantification of metal ions • Detection of metal in various samples ElectromagneticRadiation Interact with Matter (Atoms, Molecules)= Spectroscopy
- 4. Diatomic molecule of same element DON’T absorb IR • Symmetricaldiatomic bond will not absorb IR • No change in dipole moment as molecule vibrate • No absorption of IR No change in dipole moment MolecularVibration Polar molecule will absorb IR • H-CI, as bond stretches, distance bet atoms increases, results in change in dipole moment • Absorb IR Condition formolecularvibration to absorba photon /IR • Vibration causeoscillation in developinga change in dipole bet oppositecharged centres • Vibration of bond in HCI causedipole in bond to oscillate • Cause a change in dipole moment Oscillationof bonds - lead to oscillation of dipole - change in dipole moment IR absorb - Molecular Vibration – dipole moment change Change in dipole moment IR freq = Natural freq for bond – Resonance will happen. • HCI bond has natural vibrational freq • IR freq match the vibrational freq in HCI, IR is absorb and molecule excited to vibrational state • IR absorb by bond will result in greater vibration in amplitude Diatomic Molecules Vs IR frequency is applied IR frequency = Natural frequency for bond ↓ IR absorbed and resonance will happen Dipole change + -
- 5. Diff bondabsorb IR radiation at frequency/wavenumber. IR spectra organic compound with diff functional gps IR Absorption by diff bonds/functional gps IR Absorption diff functional gps and fingerprint region Bond Wavenumber/cm-1 C –CI (Halogenoalkanes) 700-800 C – O (alcohol, ether, ester) 1000 - 1300 C = C (alkene) 1610 - 1680 C = O ( carbonyl) 1680 – 1750 C ≡ C (alkynes) 2070 - 2250 O –H (H bond in COOH) 2500 - 3300 C – H (alkane, alkene) 2840 - 3095 O – H (H bond in alcohol) 3230 - 3550 N – H (amines) 3350 - 3500 C – H stretch (2840 – 3000) C – O stretch (1000-1300) C = O stretch (1680 -1740) O – H stretch (3230 -3550) C = C stretch (1610-1680) Fingerprinting region • Range (1500- 400cm-1 ) • Specific to each molecule Click here khan organic videos. Click here khan IR videos.
- 6. IR spectra organic compound with diff functional gps IR Absorption by diff bonds/functional gps Bond Wavenumber/cm-1 C –CI (Halogenoalkanes) 700-800 C – O (alcohol, ether, ester) 1000 - 1300 C = C (alkene) 1610 - 1680 C = O ( carbonyl) 1680 – 1750 C ≡ C (alkynes) 2070 - 2250 O –H (H bond in COOH) 2500 - 3300 C – H (alkane, alkene) 2840 - 3095 O – H (H bond in alcohol) 3230 - 3550 N – H (amines) 3350 - 3500 Fingerprinting region • Range (1500- 400cm-1 ) • Specific to each molecule Transmittance/%Absorbance Absorption/Transmittanceplotted twoways. Transmittance Y-axis / wavenumber X-axis. Absorption on Y-axis / wavenumber on X-axis. Trans, % (T) and Absorbance (A) Trans 100%mean IR Absorbance 0% Trans 0% mean IR Absorbance 100% wavenumber/cm-1 Transmittance 100% Absorbance 0% Transmittance 0% Absorbance 100% Infra Red Spectroscopy • Wavenumber α frequency • Wavenumber = Reciprocalof wavelength(1/λ) , Unit = cm-1 • Wavenumber= 1/Wavelength = numberwave cycles in one cm IR wavelength from (2500 – 25000)nm → Convert to wavenumber(400 – 4000) cm-1 λ = 2500 nm (convert to cm) → λ = 0.00025 cm → Wavenumber= 1/λ = 1/0.00025 = 4000 cm-1 λ = 25000 nm (convert to cm) → λ = 0.0025 cm → Wavenumber= 1/λ = 1/0.0025 = 400 cm-1 λ low ↓ → Wavenumber, 1/λ is High ↑ → f = c x 1/λ → f is High ↑ → Energy = hf High ↑ wavenumber bet 400 – 4000cm-1 Higher Wavenumber ↑ = Lower wavelength ↓= Higher ↑ frequency = GreaterEnergy↑ Click here khan wavenumber. Click here khan organic videos.
- 7. Strength of bond Single, Double, Triple Bond Mass of atom Lighter/Lower Mass atom • Higher energy frequency for vibration Stretching Vs Bending Vibration IR absorption frequency Heavier/Higher Mass atom • Lower energy/frequency for vibration Bending Vibration • Less energy need for resonance • Lower frequency/wavenumber Stretching Vibration • More energy need for resonance • Higher frequency/wavenumber Stronger bond • Higher energy need for resonance • Higher frequency/wavenumber Weaker bond • Lower energy need for resonance • Lower frequency/wavenumber IR absorptionfrequency Strong bond Weak bond C- H = 2840cm-1 C- CI = 600cm-1 C- H stretch = 2840cm-1 C- H bend = 1400cm-1 Bond Bond enthalpy Wavenumber C –C 348 800-1200 C = C 612 1610-1680 C ≡ C 837 2070-2250 Stretching Vibration Bending Vibration
- 8. Molecule to absorb IR • Vibrationwithin molecule cause a net change in dipole moment • Frequency of radiation matchesvibrational naturalfrequency of molecule, radiation will be absorbed, causing a change in amplitude of molecularvibration. • A permanent dipole not necessary, only a change in dipole moment • Not all bond absorb IR . For IR absorption, bond must have an electric dipole (bond polarity) that changes as it vibrates. • Molecules absorb IR – cause changes in modes of vibration (stretching/bending) InfraredSpectroscopyand MolecularVibration MolecularVibration StretchingMode BendingMode Symmetric Stretching • change in bond length • bond become shorter/longer • IR ACTIVE (change in dipole) • IR INACTIVE (No change in dipole) Asymmetric Stretching • change in bond length • bond become shorter/longer • IR ACTIVE (change in dipole) • IR INACTIVE (No change in dipole) Symmetric Bending • change in bond angle • bond angle bigger/smaller • IR ACTIVE (change in dipole) • IR INACTIVE (No change in dipole) Asymmetric Bending • change in bond angle • bond angle bigger/smaller • IR ACTIVE (change in dipole) • IR INACTIVE (No change in dipole) wagging twisting rocking scissoring
- 9. Molecular Vibration Stretching Mode Bending Mode Symmetric Stretching - change in bond length - bond become short/long - Change dipole moment - Absorb IR (active) at 3652 Asymmetric Stretching - change in bond length - bond become short/long - change dipole moment - Absorb IR (active) at 3756 Symmetric Bending - change in bond angle - Angle bigger/smaller - change dipole moment - Absorb IR (active) at 1595 Molecular Vibrationfor H2O(IR Spectrum) IR spectrum for H2O Molecular Vibrationfor SO2 (IR Spectrum) Molecular Vibration Stretching Mode Symmetric Stretching - change in bond length - bond become short/long - Change dipole moment - Absorb IR (active) at 1150 Asymmetric Stretching - change in bond length - bond become short/long - change dipole moment - Absorb IR (active) at 1360 IR spectrum for SO2 Click here Spectra database (Ohio State) Click here Spectra database (NIST)
- 10. Molecular Vibration Stretching Mode Bending Mode Symmetric Stretching - Bond polarity cancel out - bond become short/long - NO Change dipole moment - IR (inactive) Asymmetric Stretching - change in bond length - bond become short/long - change dipole moment - Absorb IR (active) at 2349 Symmetric Bending - change in bond angle - Angle bigger/smaller - change dipole moment - Absorb IR (active) at 667 Molecular Vibrationfor CO2 (IR Spectrum) IR spectrum for CO2 Molecular Vibrationfor SO2 (IR Spectrum) Molecular Vibration Stretching Mode Symmetric Stretching - change in bond length - bond become short/long - Change dipole moment - Absorb IR (active) at 1150 Asymmetric Stretching - change in bond length - bond become short/long - change dipole moment - Absorb IR (active) at 1360 IR spectrum for SO2 Click here Spectra database (Ohio State) Click here Spectra database (NIST)
- 11. Propanal (CH3CH2CHO) • (2840-3000)→ C-H stretch • (2720) → C-H stretch CHO • (1680-1740) → C=O stretch Hex-1-ene CH2=CH(CH2)3CH3 • (2840-3000) → C-H stretch • (1610-1680) → C = C stretch • (1200- 1400) → C-H bend Hex-1-yne CH2≡CH(CH2)3CH3 • (3350) → C ≡ C stretch • (2840-3000)→ C-H stretch • (1200- 1400) → C-H bend IR spectra organic compoundwith diff functional gps Chloromethane CH3CI • (2840-3000)→ C-H stretch • (1200-1400)→ C-H bend • (700-800) → C-CI stretch Halogenoalkane Aldehyde Alkene Alkyne C – H stretch (2840 – 3000) C – H bend (1200) C – CI stretch (700-800) C – H stretch CHO (2720) C = O stretch (1680 – 1740) C – H stretch (2840 – 3000) C = C stretch (1610-1680) C – C bend C ≡ C stretch (3350) C – H stretch (2840 – 3000) C – H bend (1200) CH3CI CH3CH2CHO CH2=CH(CH2)3CH3 CH2≡CH(CH2)3CH3 C – H stretch (2840 – 3000)
- 12. Methanol (CH3OH) • (3230-3550) → O-H stretch • (2840-3000) → C-H stretch • (1000-1300) → C-O stretch Ethanol(CH3CH2OH) • (3230-3550) → O-H stretch • (2840-3000) → C-H stretch • (1000-1300) → C-O stretch Phenol (C6H5OH) • (3230-3550) → O-H stretch • (2840-3000) → C-H stretch • (1400-1500) → C=C aromatic stretch • (1000-1300) → C-O stretch Benzoic acid (C6H5COOH) • (3230-3550) → O-H stretch • (2840-3000) → C-H stretch • (1400-1500) → C=C aromatic stretch • (1000-1300) → C-O stretch • (1680-1740) → C=O stretch IR spectra organic compoundwith diff functional gps C – O stretch (1000-1300) C – H stretch (2840 – 3000) O – H stretch (3230 -3550) Broad Absorption due to H bonding bet molecules Broad Absorption due to H bonding bet molecules CH3OH CH3CH2OH C – O stretch (1000-1300) O – H stretch (3230 -3550) C – H stretch (2840 – 3000) O – H stretch (3230 -3550) C – H stretch (2840 – 3000) C – O stretch (1000-1300)C = O stretch (1680 – 1740) C = C stretch (1610-1680) Broad Absorption due to H bonding bet molecules O – H stretch (3230 -3550) C – H stretch (2840 – 3000) C = C stretch (1610-1680) C – O stretch (1000-1300) Broad Absorption due to H bonding bet molecules
- 13. Spectra diff bet Acid and Ester Ethyl ethanoate(CH3COOCH2CH3) • (2840-3000)→ C-H stretch • (1680-1740) → C=O stretch • (1000-1300) → C-O stretch Methanoic acid (HCOOH) • (3230-3550) → O-H stretch • (2840-3000) → C-H stretch • (1000-1300) → C-O stretch • (1680-1740) → C=O stretch Methanoic acid (HCOOH) • (3230-3550) → O-H stretch • (2840-3000) → C-H stretch • (1000-1300) → C-O stretch • (1680-1740) → C=O stretch Methanoic acid Methanoic acid Spectra diff bet Acid and Alcohol Methanol (CH3OH) • (3230-3550) → O-H stretch • (2840-3000) → C-H stretch • (1000-1300) → C-O stretch Ethyl Ethanoate Methanol Vs Vs O – H stretch (3230 -3550) C – H stretch (2840 – 3000) C – O stretch (1000-1300) C = O stretch (1680 -1740) C – H stretch (2840 – 3000) C = O stretch (1680 - 1740) C – O stretch (1000 - 1300) O – H stretch (3230 - 3550) C – H stretch (2840 – 3000) C = O stretch (1680 - 1740) C – O stretch (1000 - 1300) CH3OH O – H stretch (3230 -3550) C – H stretch (2840 – 3000) C – O stretch (1000-1300)
- 14. Propan -2-ol CH3CH(OH)CH3 Propanone CH3COCH3 Hexan-1-ol CH3(CH2)4CH2OH Hexan-2-one CH3CO(CH2)3CH3 C - H ↓ ← C-H bend C-H bend → Vs Vs Spectra diff bet Alcohol and Ketone OH ↓ C - H ↓ ← C=O ← C-H bend ↑ C - H ← C-H bend Spectra diff bet Alcohol and Ketone
- 15. ← C-H stretch ← C-H stretch ← C-H stretch ← C-H stretch ← C=O stretch C=O stretch → O-H ↓ O-H ↓ ← C-H bend ← C-H bend C-H bend → C-H bend → C=C stretch → Finger printing region Finger printing region Finger printing region Finger printing region Propanal (Aldehyde) Butan-2-one (Ketone) Butanol (Alcohol) But-2-en-1-ol( Alcohol + Alkene) IR spectra organic compound with diff functional gps C – H stretch CHO (2720) H ׀ CH3-CH2-C=O O ‖ CH3CH2-C-CH3 CH3-CH=CH-CH2-OHCH3-CH2-CH2-CH2OH
- 16. Operating Principle Double Beam Infrared Spectrometer Double beam splitter • Direct half radiation through sample and otherhalf through reference • Allow radiation passing through sample and compare it with reference • Two beamsrecombinedat detector. • Signal from sample/reference are compared to determine if sample absorbradiation emittedfrom source Reference • Solvent to dissolve sample • Reference use to eliminate instrument fluctuation, absorption due to impurities in solvent and all interferences. • IR Absorption due to solute using the reference Monochromator • Allow radiation of particularwavelength to pass through Fouriertransformation • Allow several wavelength through the sample at the same time and analyse the results • Using mathematicaltechniques to determinethe amplitude/intensityof each single frequency • Fouriertransformation-Intensityof IR radiation at each frequencydeterminedseparately Recorder/Output •Scanningwavenumber from 400 to 4000cm-1 • Spectrum of Abs/Trans vs frequency/wavenumber produced Light Source • Provide IR radiation
