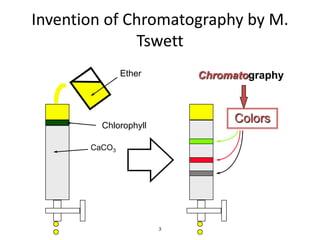
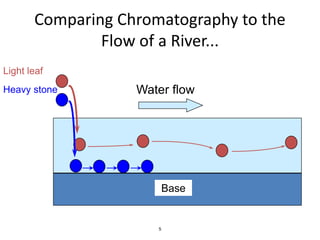
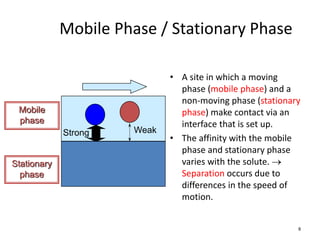
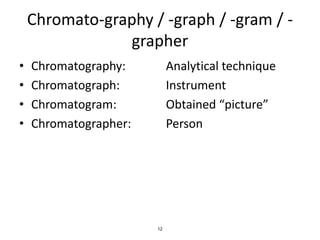
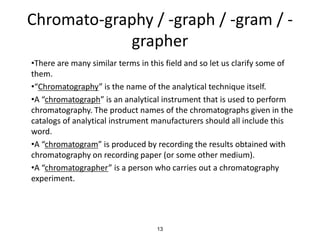
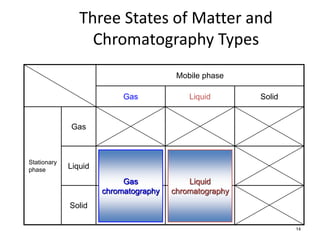
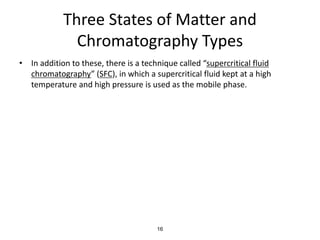
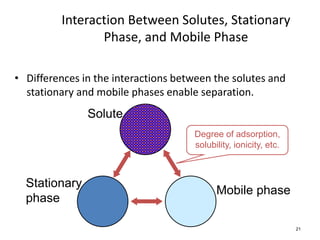
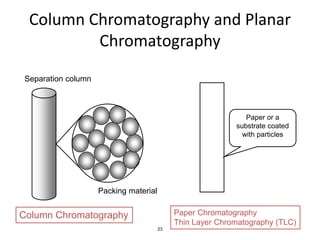
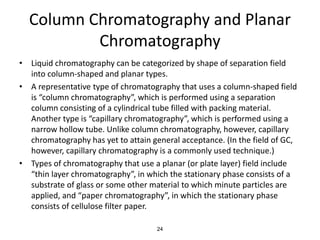
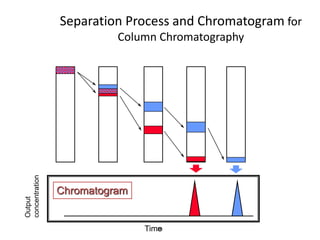
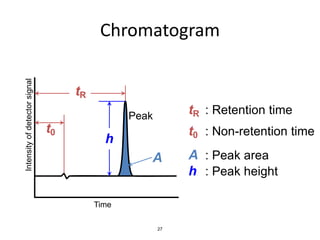
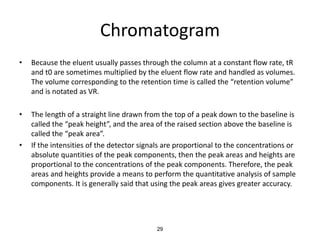
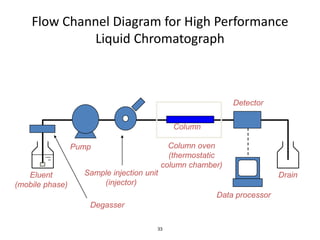
HPLC is a type of chromatography that uses high pressure to force a liquid mobile phase through a column packed with solid particles. This allows for faster analysis times and better separation of components compared to traditional liquid chromatography. HPLC systems include a pump to deliver the mobile phase, an injector for samples, a column inside an oven, a detector, and a data processor. The interaction of sample components with the stationary and mobile phases causes separation as components move through the column at different speeds.