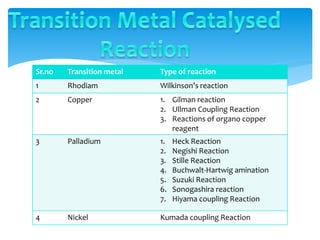
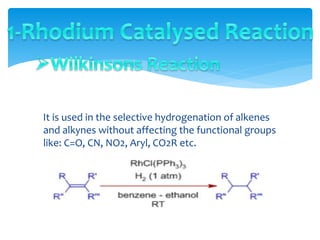
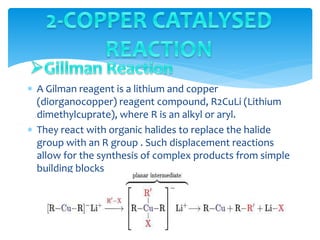
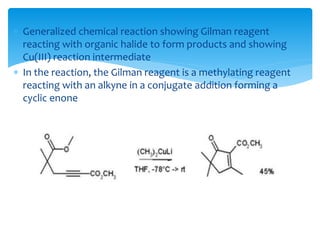
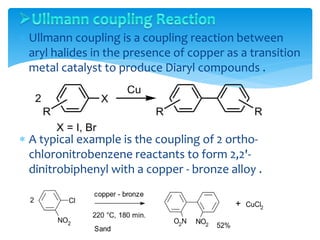
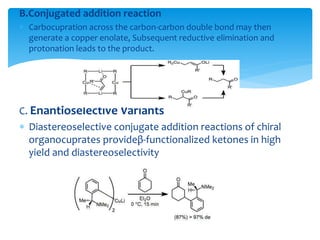
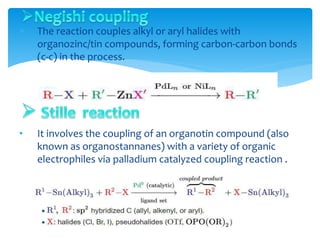
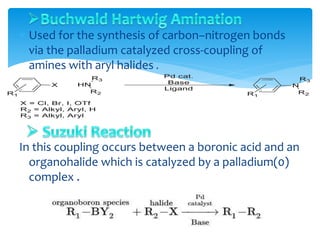
Transition metals are defined by their partially filled d orbitals, enabling them to act as effective catalysts in various reactions due to their ability to form compounds in multiple oxidation states. The document outlines various reactions facilitated by transition metals and specific catalysts, including the Gilman reagent, Ullmann coupling, and the Sonogashira reaction, highlighting their roles in organic synthesis. Additionally, it discusses the factors contributing to the effectiveness of transition metals in catalysis, such as bonding ability and variable coordination numbers.


![Zinc, cadmium, and mercury are generally excluded
from the transition metals as they have the electronic
configuration [ ]d 10s 2 , with no incomplete d shell.](https://image.slidesharecdn.com/52-191219093816/85/TRANSITION-METAL-CATALYSIS-3-320.jpg)