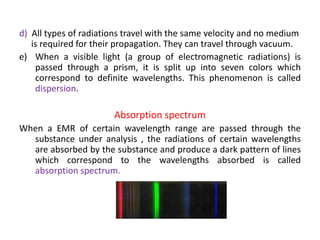
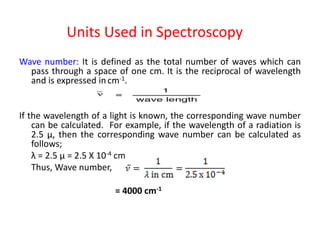
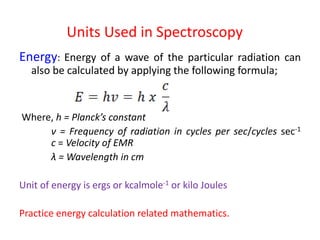
Electromagnetic radiation is produced by the oscillation of electric and magnetic fields residing on atoms. They are characterized by their wavelength, frequency, or wave number and travel at the speed of light. When visible light passes through a prism, it disperses into the colors of the visible spectrum corresponding to different wavelengths. In absorption spectroscopy, when radiation of a certain wavelength range passes through a substance, specific wavelengths are absorbed, producing a dark-line absorption spectrum corresponding to the wavelengths absorbed.