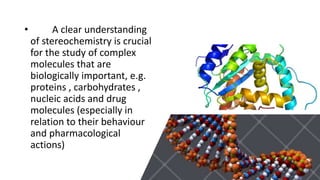
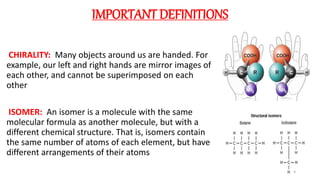
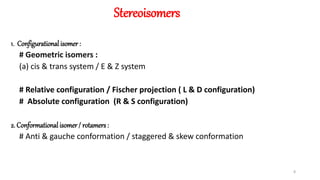
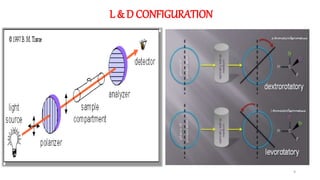
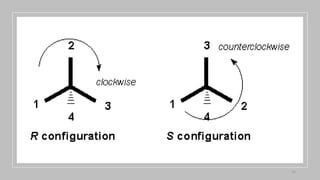
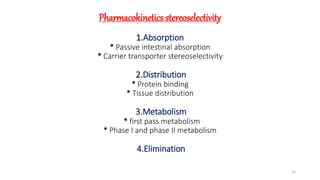
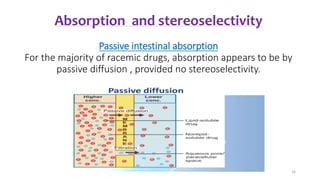
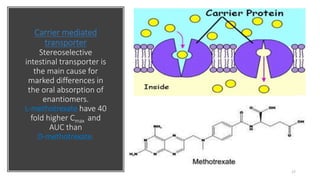
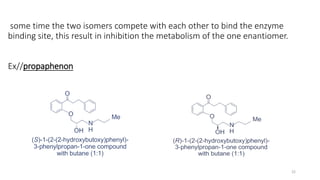
The document discusses the importance of stereochemistry in understanding biologically significant molecules such as drugs, proteins, and nucleic acids. It covers key concepts including chirality, isomers, stereoisomers, and the impact of stereochemistry on pharmacokinetics and pharmacodynamics of drugs, highlighting how different enantiomers can exhibit varied biological effects. It also presents examples of stereoselectivity in drug absorption, distribution, metabolism, and elimination based on differences between enantiomers.