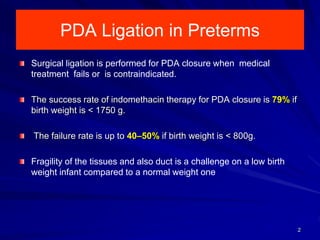
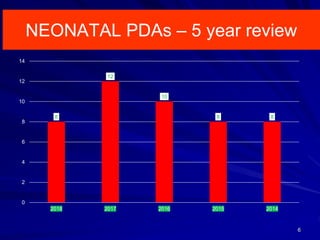
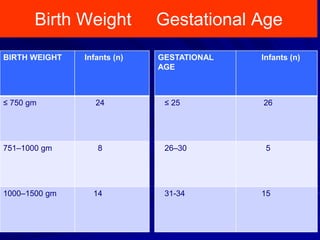
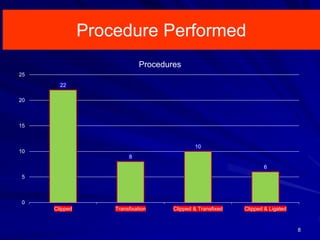
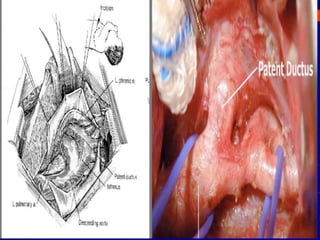
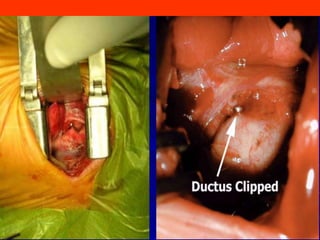
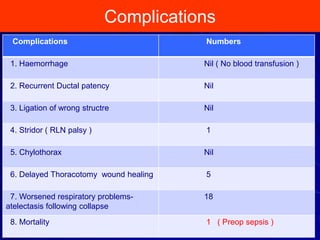
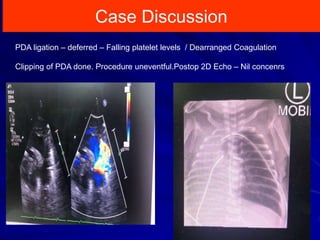
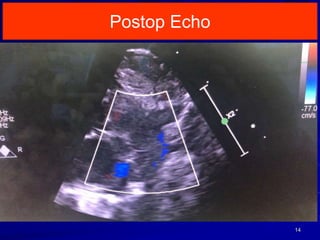
This document discusses surgical closure of patent ductus arteriosus (PDA) in premature infants. It provides guidelines for when surgical referral is appropriate, such as when two courses of medication have failed to close a large PDA. The success rate of medication closure is 79% for infants over 1750g and lower for infants under 800g. The document outlines pre-operative testing and guidelines for the surgery including echocardiogram, bloodwork and discussions between medical teams. Potential complications are discussed such as hemorrhage, recurrent ductal patency and respiratory problems. Post-ligation cardiac syndrome is also summarized, which can involve low blood pressure, increased ventilation needs, and interventions like inotropic support.